Linear (d-π-a-π)2-d type organic small molecule photovoltaic materials containing benzotriazole units and their preparation and application
A technology of benzotriazole and photovoltaic materials, which is applied in the field of organic small molecule photovoltaic materials and its preparation, can solve the problems of low overall efficiency of solar cells, and achieve the effect of improving solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
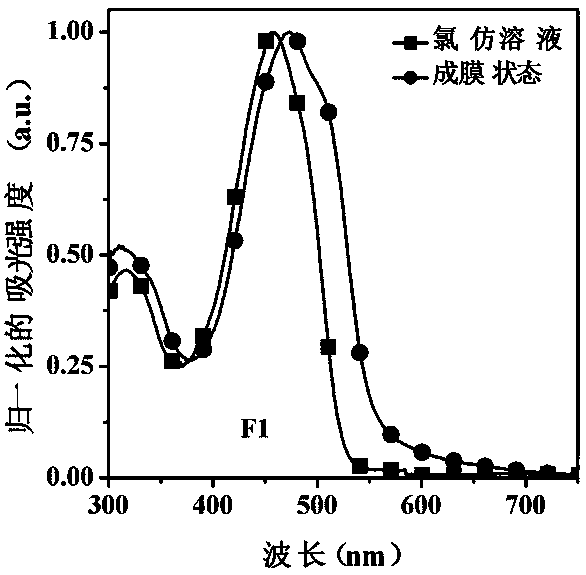
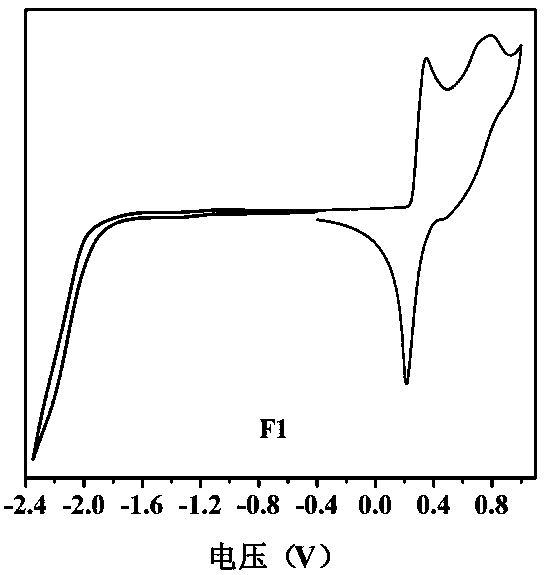
[0038] This example discloses the linear (D-π-A-π) 2 -The specific synthesis process of D-type organic small molecule donor material F1 includes the following steps:
[0039]Under nitrogen protection, compound M2 (122 mg, 0.1 mmol), N-(4-(4,4,5,5-tetramethyl-[2,1,3]dioxaborolyl)benzene base)-N-phenylaniline ie Q represents D2 when C-C (93 mg, 0.25 mmol), potassium carbonate (552 mg, 4.0 mmol) and tetrakis(triphenylphosphine)palladium (11 mg, 0.01 mmol) were dissolved in toluene (4 mL), deionized water (2 mL) and ethanol (1 mL), and reflux at 110°C for 18-24 hours. After the reaction mixture was cooled to room temperature, it was poured into 20 mL deionized water for separation, and extracted with dichloromethane. The organic phases were combined and dried over anhydrous sodium sulfate. The organic solvent was removed by rotary evaporation, and the crude product was purified by column chromatography using petroleum ether / dichloromethane (v:v, 3:1) as a developing solvent to ...
Embodiment 2
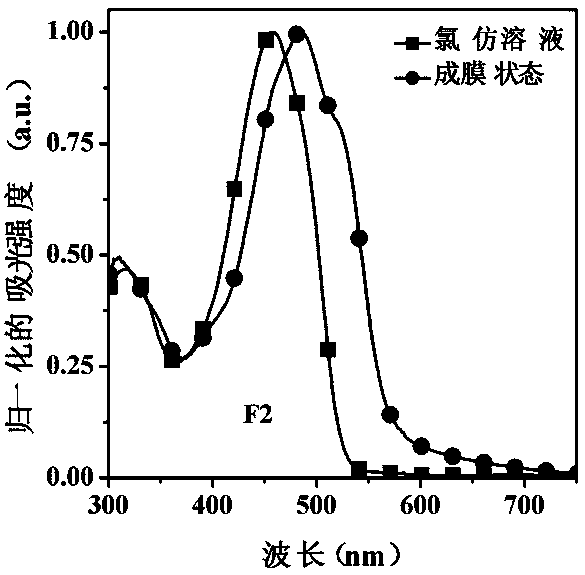
[0046] This example discloses the linear (D-π-A-π) 2 -The specific synthesis process of D-type organic small molecule donor material F2 includes the following steps:
[0047] Under the protection of nitrogen, the compound M2 (122 mg, 0.1 mmol), 4-(N,N-diphenylamino) styrene, that is, D2 when Q represents C=C (68 mg, 0.25 mmol), palladium acetate (2 mg, 0.01 mmol), sodium acetate (205 mg, 2.5 mmol) and TBAB (13 mg, 0.04 mmol) were dissolved in N,N-dimethylformamide (10 mL) solution, and refluxed at 100°C for 36 hours . After the reaction solution was cooled to room temperature, it was poured into 50 mL deionized water and extracted with dichloromethane, and then the organic phase was washed with deionized water. Finally the organic phase was dried over anhydrous sodium sulfate. After rotary evaporation to remove the organic solvent, the crude product was purified by column chromatography using petroleum ether / dichloromethane (v:v, 5:2) as the developing solvent to obtain a r...
Embodiment 3
[0054] This example discloses the linear (D-π-A-π) 2 -The specific synthesis process of D-type organic small molecule donor material F3 includes the following steps:
[0055] Compound M2 (244 mg, 0.2 mmol), 4-(N,N-diphenylamino) phenylacetylene, that is, D2 (134 mg, 0.5 mmol) when Q represents C≡C, bis(triphenylphosphine) di Palladium chloride (4 mg, 0.005 mmol) and cuprous iodide (2 mg, 0.01 mmol) were dissolved in a mixed solution of tetrahydrofuran (20 mL) and triethylamine (15 mL), and reacted under reflux at 70°C for 24 hours. After the reaction solution was cooled to room temperature, it was poured into 50 mL of deionized water and extracted with dichloromethane. The organic phases were combined and dried over anhydrous sodium sulfate. After rotary evaporation to remove the organic solvent, the crude product was purified by column chromatography using petroleum ether / dichloromethane (v:v, 5:2) as the developing solvent to obtain the target product as an orange-red solid...
PUM
| Property | Measurement | Unit |
|---|---|---|
| open-circuit voltage | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com