Sulfide-containing industrial wastewater treatment reagent and reaction method thereof
A technology of industrial wastewater and sulfide, applied in the chemical field, can solve the problems of incomplete degradation of organic pollutants, low utilization rate of hydrogen peroxide, increased water treatment costs, etc., and achieve the effect of broadening the application range, reducing the dosage, and reducing the treatment cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
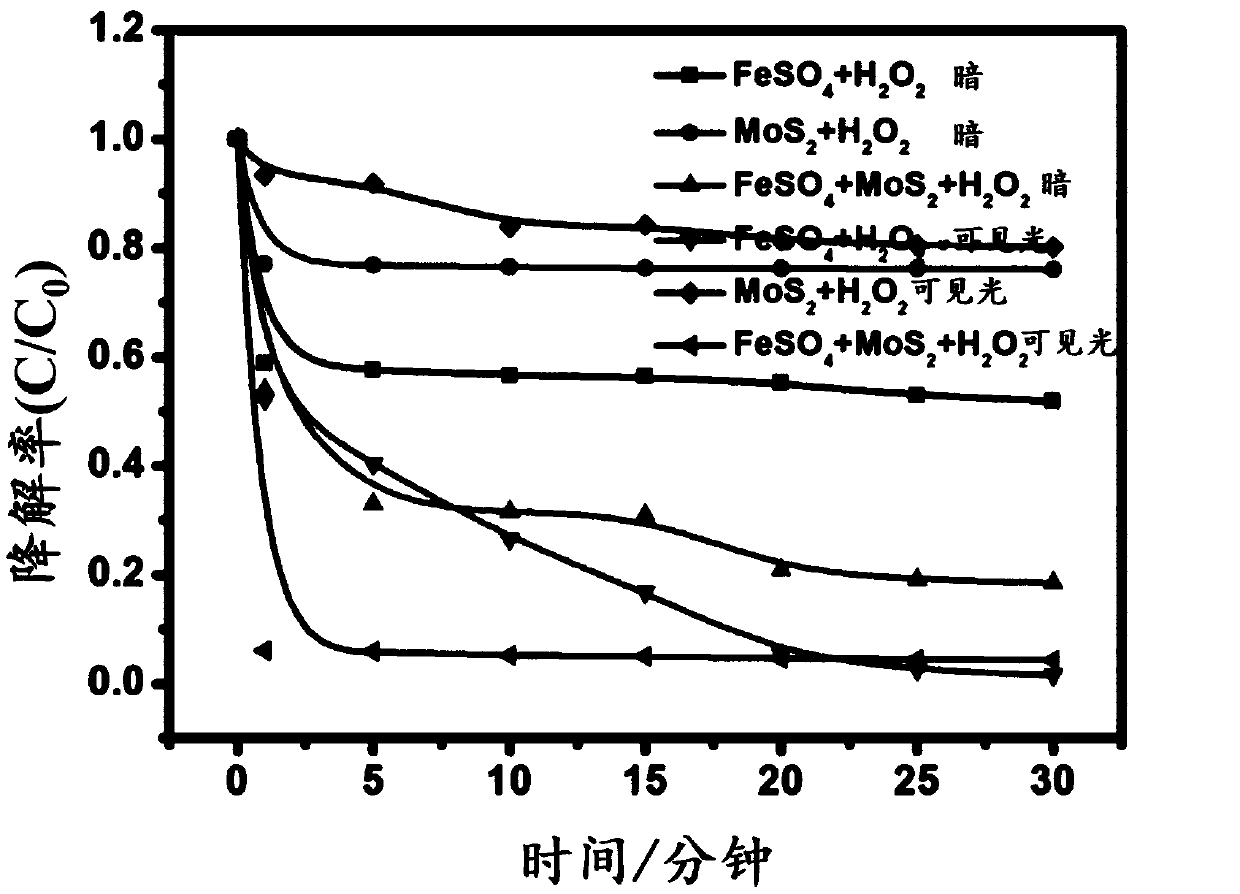
[0047] The control experiment was carried out according to the following six groups of conditions,
[0048] (1) 100L of waste water to be treated, FeSO 4 Concentration 0.02g / L, H 2 o 2 Concentration 0.4mmol / L, no visible light;
[0049] (2) 100L of waste water to be treated, MoS 2 Concentration 0.3g / L, H 2 o 2 Concentration 0.4mmol / L, no visible light;
[0050] (3) Wastewater to be treated 100L, FeSO 4 Concentration 0.02g / L, MoS 2 Concentration 0.3g / L, H 2 o 2 Concentration 0.4mmol / L, no visible light;
[0051] (4) Wastewater to be treated 100L, FeSO 4 Concentration 0.02g / L, H 2 o 2 Concentration 0.4mmol / L, with visible light;
[0052] (5) Wastewater to be treated 100L, MoS 2 Concentration 0.3g / L, H 2 o 2 Concentration 0.4mmol / L, with visible light;
[0053] (6) Wastewater to be treated 100L, FeSO 4 Concentration 0.02g / L, MoS 2 Concentration 0.3g / L, H 2 o 2 Concentration 0.4mmol / L, with visible light;
[0054] Experimental results such as figure 1 shown...
Embodiment 2
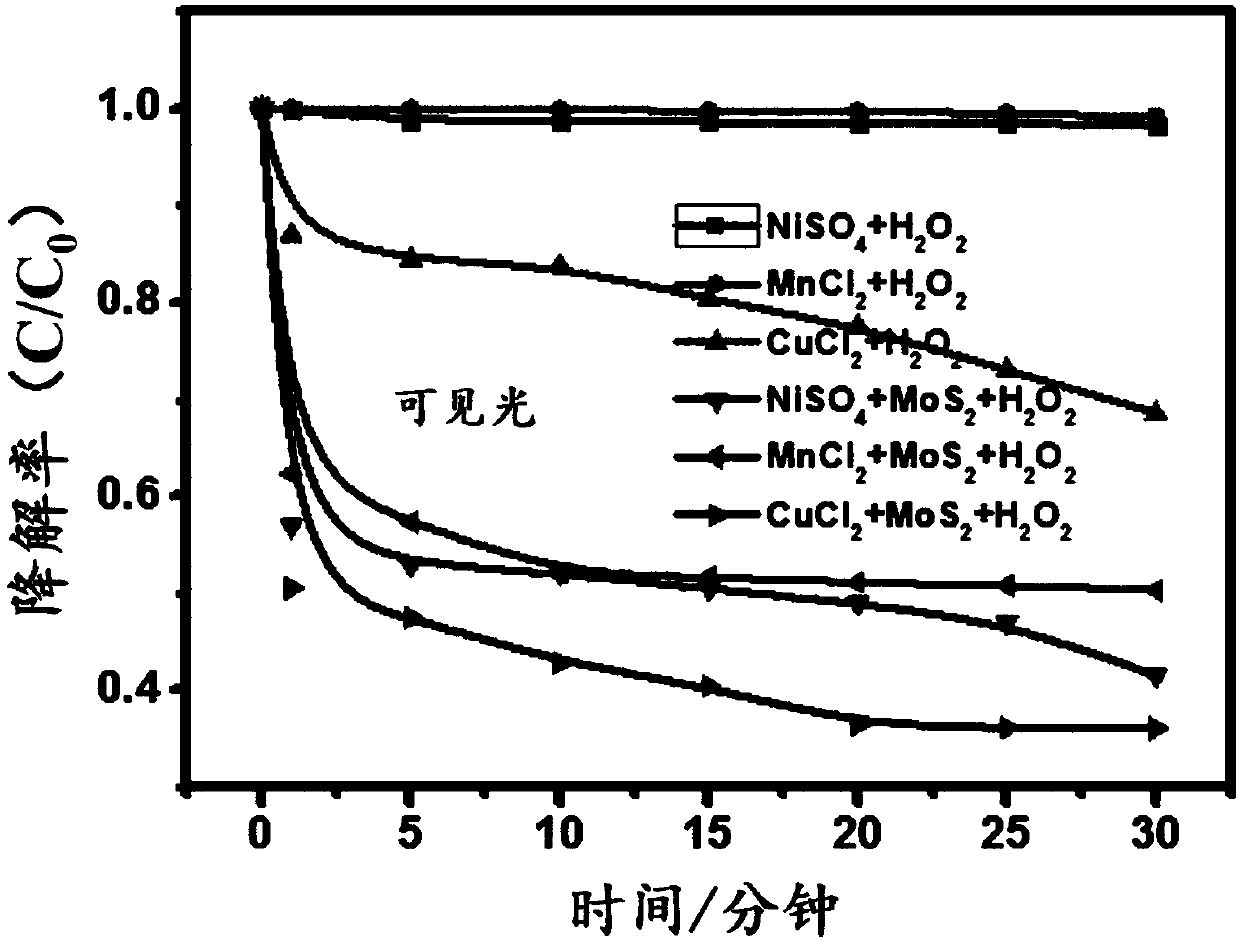
[0058] According to the following six groups of conditions for comparative experiments, the following six groups of conditions are all illuminated with visible light,
[0059] (1) 100mL of waste water to be treated, NiSO 4 Concentration 0.02g / L, H 2 o 2 Concentration 0.4mmol / L;
[0060] (2) 100mL of waste water to be treated, MnCl 2 Concentration 0.02g / L, H 2 o 2 Concentration 0.4mmol / L;
[0061] (3) 100mL of waste water to be treated, CuCl 2 Concentration 0.02g / L, H 2 o 2 Concentration 0.4mmol / L;
[0062] (4) 100mL of waste water to be treated, NiSO 4 Concentration 0.02g / L, MoS 2 Concentration 0.3g / L, H 2 o 2 Concentration 0.4mmol / L;
[0063] (5) 100mL of waste water to be treated, MnCl 2 Concentration 0.02g / L, MoS 2 Concentration 0.3g / L, H 2 o 2 Concentration 0.4mmol / L;
[0064] (6) Wastewater to be treated 100mL, CuCl 2 Concentration 0.02g / L, MoS 2 Concentration 0.3g / L, H 2 o 2 Concentration 0.4mmol / L;
[0065] Experimental results such as figure 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com