Method for preventing metal corrosion through graphene depending on crystal surface
A technology of metal corrosion and graphene, which is applied in the field of crystal plane-dependent graphene protection against metal corrosion, can solve problems such as corrosion, and achieve the effects of cost reduction, low cost, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Embodiment one: a kind of method that the graphene protection metal corrosion of crystal face depends on, comprises the steps:
[0018] (1), graphene is grown on the surface of copper foil by atmospheric pressure chemical vapor deposition;
[0019] (2) Place the copper foil sample covered with graphene in the atmosphere for natural oxidation for 1 month to 2 years;
[0020] (3) After the sample is naturally oxidized, observe directly with an optical microscope, and you can see the protective effect of graphene on copper foil.
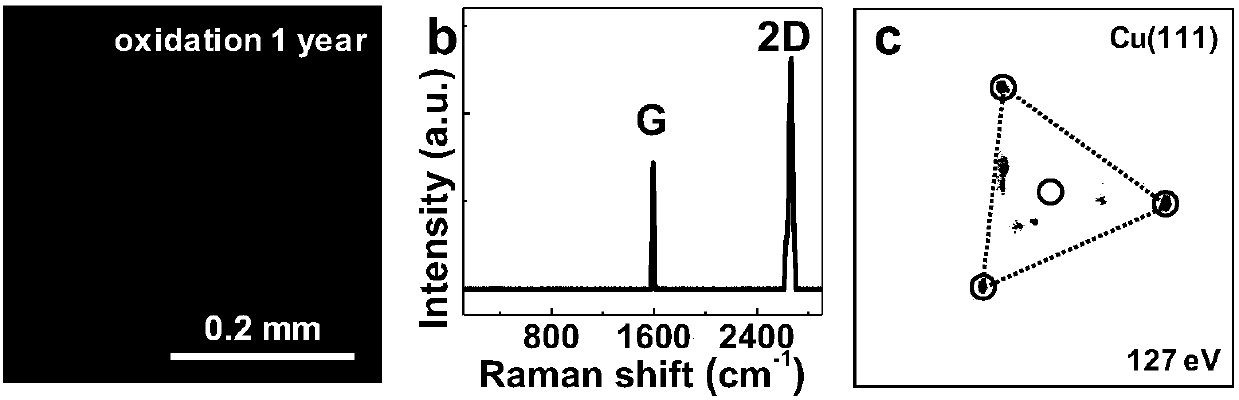
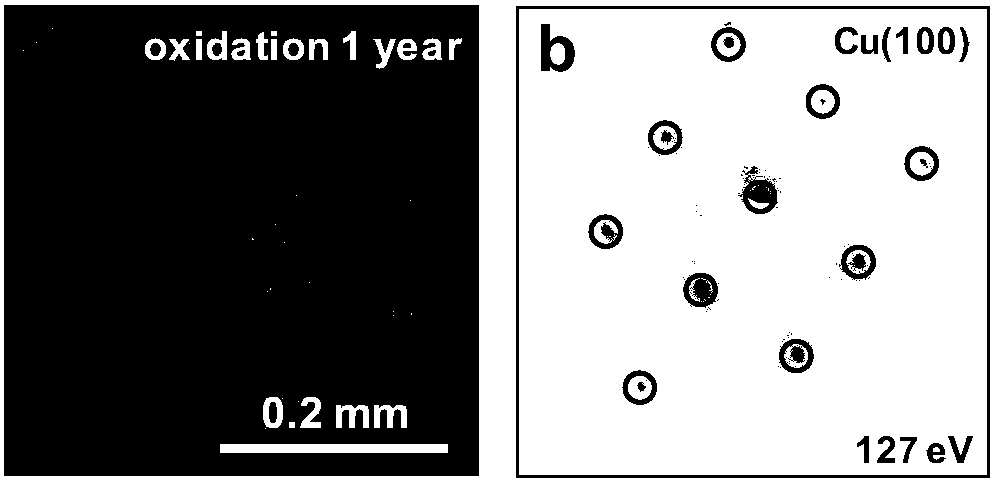
[0021] Among them, graphene is grown on the metal foil by chemical vapor deposition, or covered on the surface of the metal foil by transfer. The metal foil includes copper foil, nickel foil, etc., preferably copper foil. The specific copper crystal planes are Cu(111), Cu(311) and all crystal planes that are better matched to the graphene lattice.
[0022] The above-prepared foil covered with the Cu(111) crystal plane of graphene was placed in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com