Application of NDGA analogue to preparation of antioxidant drug
An analog, antioxidant technology, applied in drug combinations, antidote, pharmaceutical formulations, etc., can solve the problems of unseen and unseen Chinese patents on the antioxidant activity of NDGA derivatives, achieve a good dose-response relationship, inhibit ROS and other problems. Aggregate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The synthesis of embodiment 1 compound
[0030] Dissolve 3,4-dihydroxybenzaldehyde (10mmoL) and appropriate ketone (5mmoL) in ethanol, stir at room temperature, and inject HCl (gas) into the solution as a catalyst. Wherein 4-piperidone hydrochloride hydrate (1f) and 3,4-dihydroxybenzaldehyde are dissolved in a mixed solvent of ethanol:water (10:1). The progress of the reaction was monitored by thin-layer chromatography. After the reaction, the crude mixture was cooled and poured into ice water (20mL), the precipitate was precipitated, filtered by suction, and after vacuum drying, purified by silica gel column chromatography to obtain the desired product 3a- 3m, these compounds and their physicochemical properties are described below:
[0031] Effective compound 3a: (2Z,5E)-2,5-bis(3,4-dihydroxybenzylidene)cyclopentano ne(3a): Green powder, 53.6% yield, mp>300℃. 1H-NMR (600MHz, d-DMSO), δ: 9.563(s, 2H, 3-OH×2), 9.211(s, 2H, 4-OH×2), 7.238(s, 2H, Ar-CH=C ×2), 7.113(d, ...
Embodiment 2
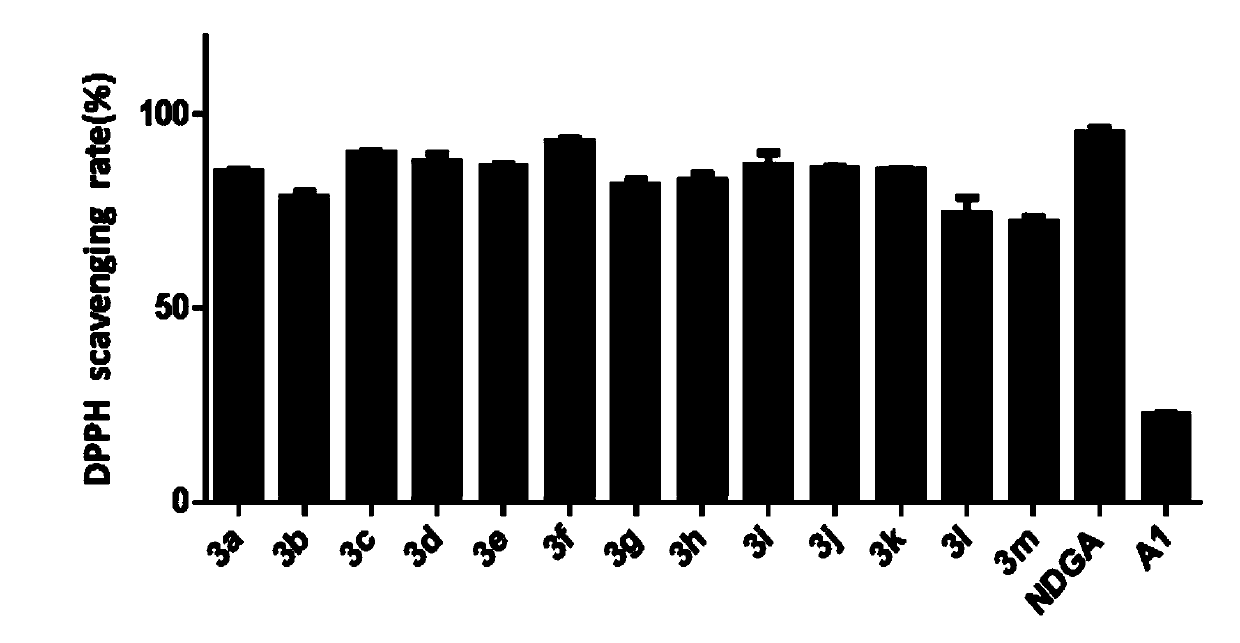
[0045] The scavenging ability of embodiment 2 compound to DPPH
[0046] The compound's ability to scavenge DPPH·free radicals was used to initially test the in vitro antioxidant activity of the compound, and the specific method was as follows: NDGA analog solution (20 mg / mL) was prepared with ethanol. DPPH·solution (0.15 mM) was also prepared in ethanol. 80 μL of NDGA analog solution was added to 120 μL of DPPH·solution (0.15 mM) (Ai). The control group was 80 μL NDGA analogue solution added to 120 μL ethanol solution (Aj). In the blank group, 120 μL DPPH·solution (Ac) was added to 80 μL ethanol solution. These mixtures were incubated at 25°C for 30 min before absorbance was measured at 517 nm. Experiments were repeated three times. Calculation of the scavenging capacity of DPPH: %=[1-(Ai-Aj) / Ac]×100. The positive drug NDGA was used as a control during the test. For the scavenging ability of compounds on DPPH, see figure 1 . It can be found that all the compounds from ...
Embodiment 3
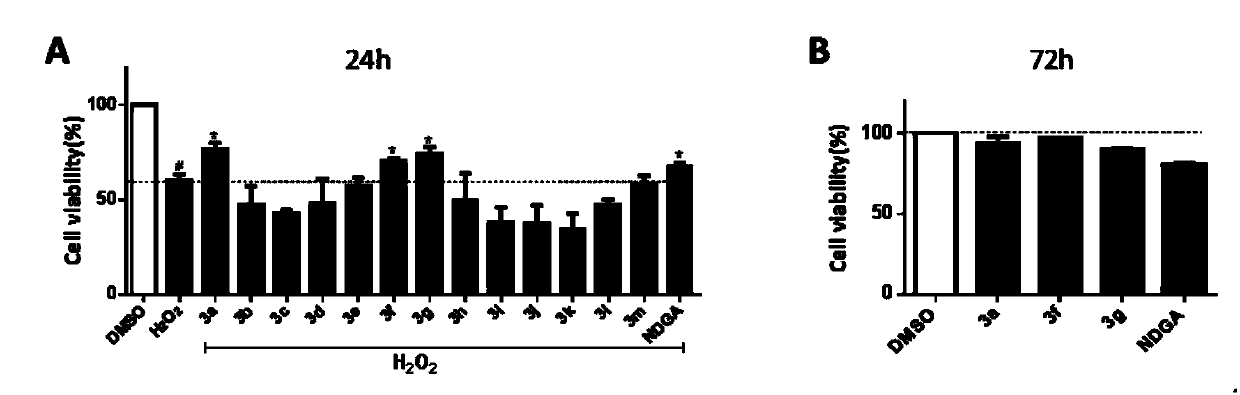
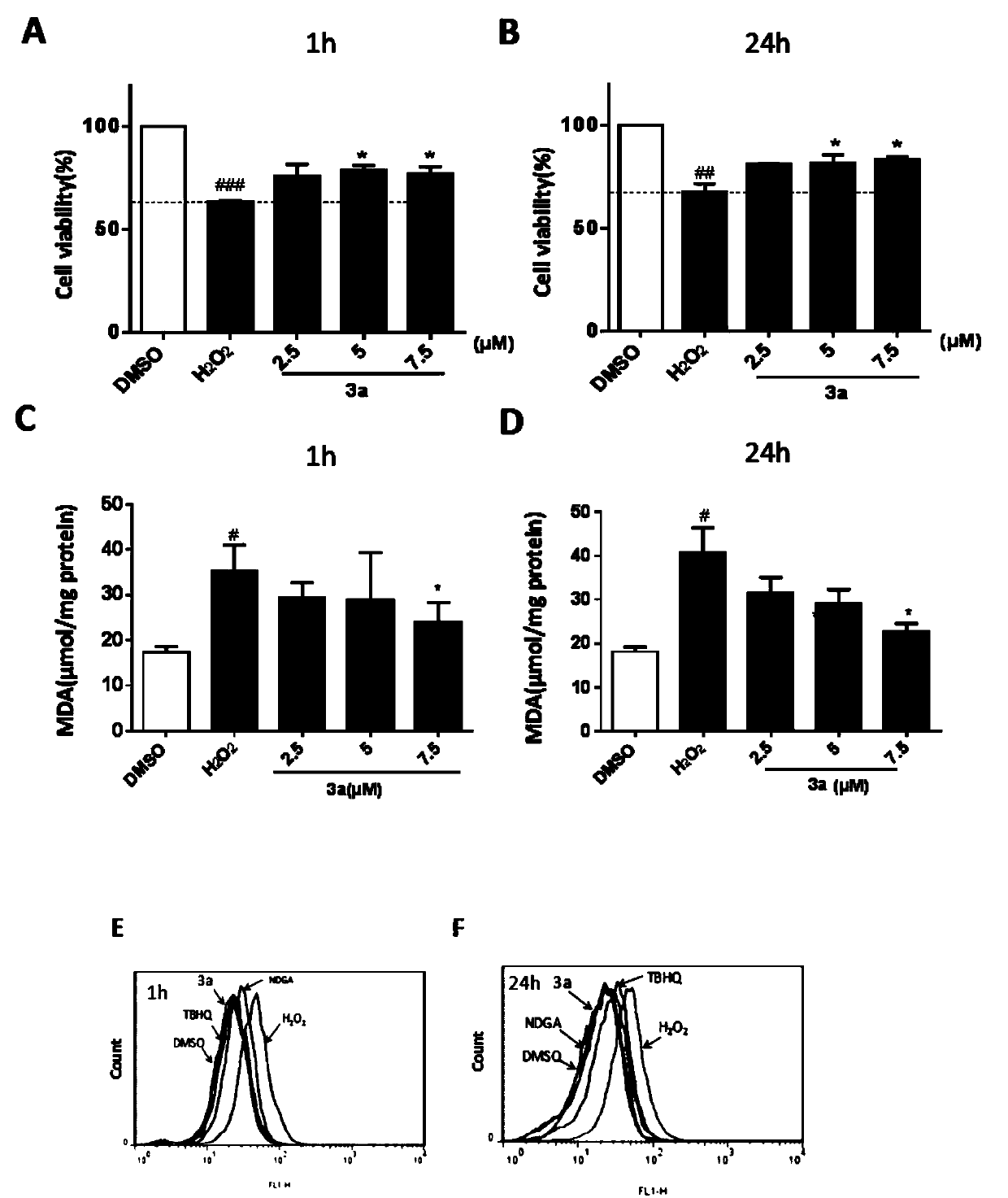
[0047] Example 3 compound to H 2 o 2 Protective effect of induced PC12 cell injury model and cytotoxicity of three active compounds in PC12 cells
[0048] to H 2 o 2 Protective effect method for inducing PC12 cell damage: PC12 cells (5 × 10 3 cells / well) were seeded in a 96-well plate, cultured in DMEM medium in a 37°C incubator, and left overnight. After adding the compound for 24 h, additional H was added 2 o 2 (400μM) injury for 24h. After 24 hours, the cells were treated with MTT solution (5 mg / mL) at 37° C. for 4 hours. Finally, formazan crystals were dissolved in 120 μL DMSO, and the OD value was measured at 490 nm. Cell viability is the percentage of the OD value of the DMSO control group. Each compound was repeatedly tested 3 times, and the average value and error value were calculated. For the experimental data, see figure 2 a.
[0049] The cytotoxicity detection method of the compound: the method is basically the same as before, the cells are also inocula...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com