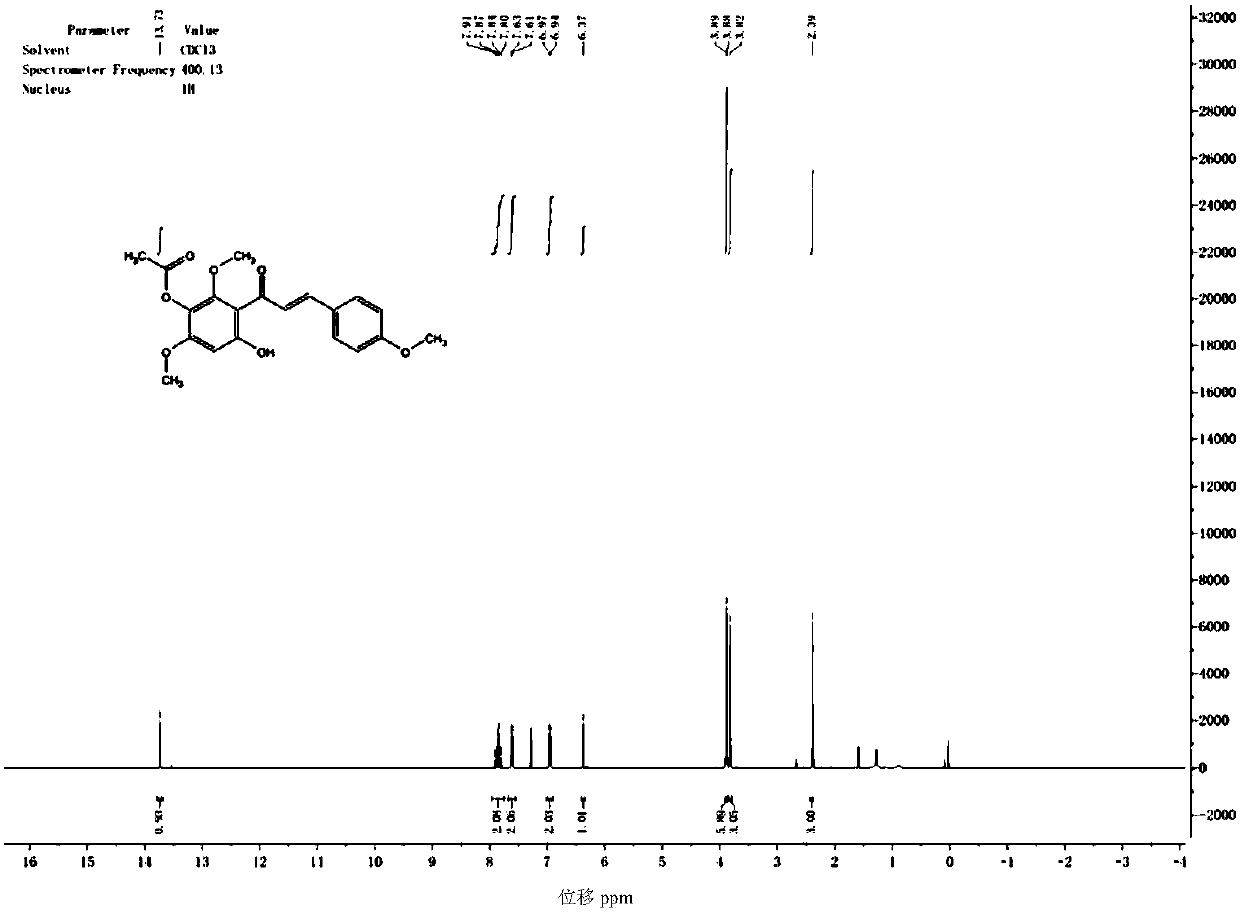
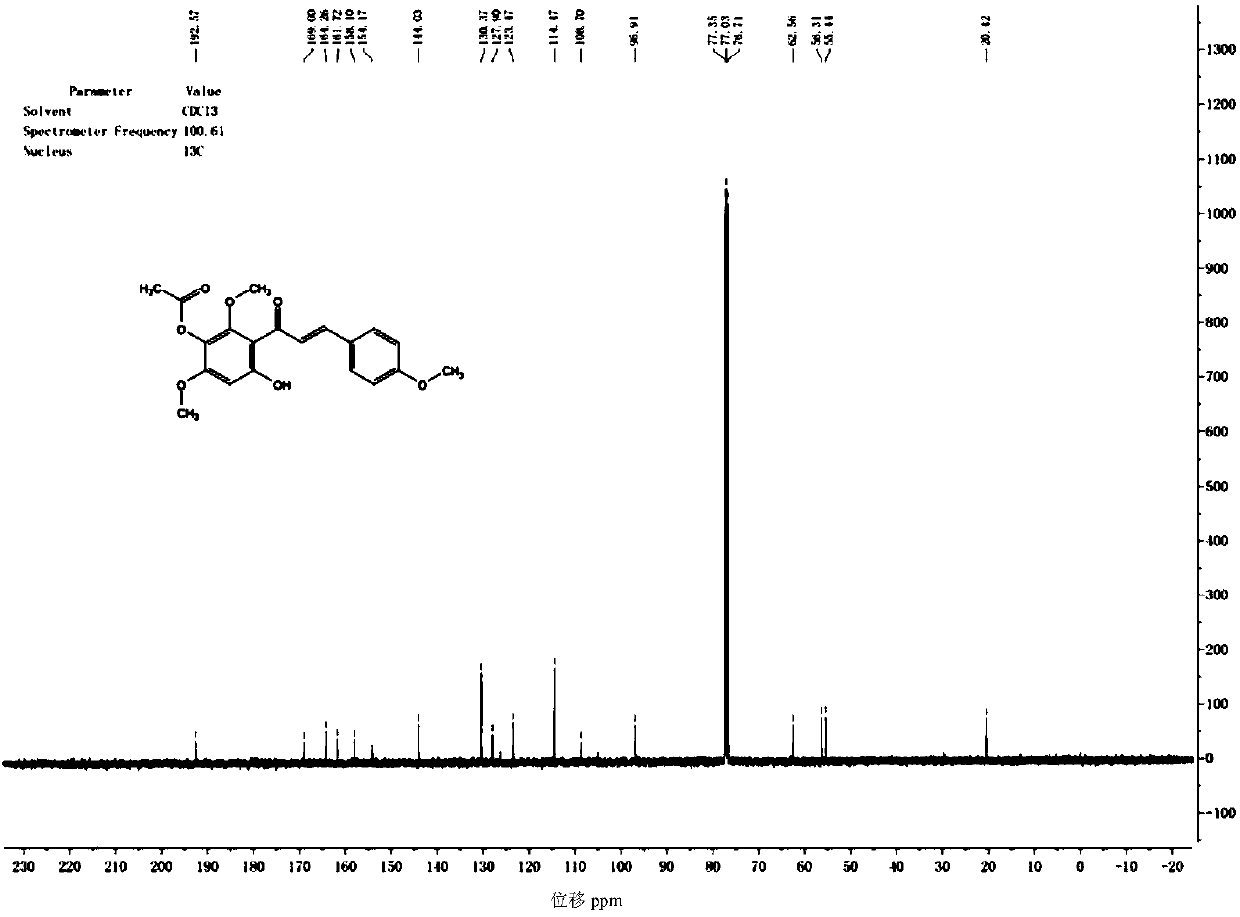
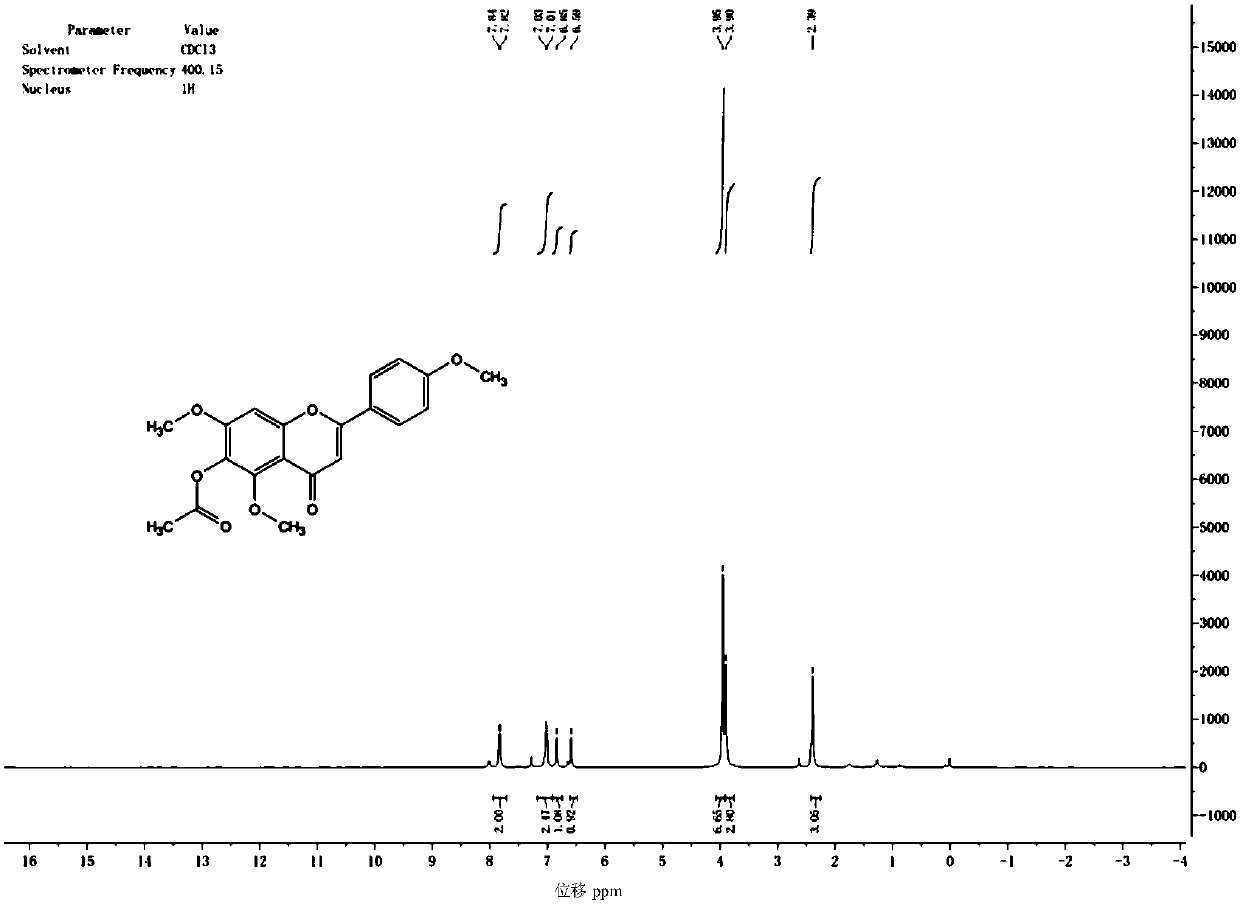
Synthetic method for scutellarein
A technique for the synthesis of aglycone aglycone, which is applied in the fields of chemical instruments and methods, the preparation of organic compounds, and the preparation of sugar derivatives, and can solve the problems of increased cost, difficult preparation, and increased difficulty
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0196] Synthesis of 2,6-dimethoxybenzoquinone (2-4)
preparation example 1
[0198]
[0199] In a 500mL Erlenmeyer flask, K 3 Fe(CN) 6 (2.0g, 7.0mmol) was dissolved in water (10.0g), and acetone (100g) and 1,3,5-trimethoxybenzene (2-3) (16.4g, 100mmol) were added to it, and stirred at 25°C After that, add H to it 2 o 2 (30%, 34g, 300mmol), stirred at 25°C for 20h, a yellow-green solid was precipitated, water (200mL) was added to the above reaction system, and 13.8g of a yellow solid was obtained by suction filtration, yield 82%, m.p.252-255°C.
preparation example 2
[0201] In a 500mL Erlenmeyer flask, K 3 Fe(CN) 6 (2.0g, 7.0mmol) was dissolved in water (10.0g), and acetonitrile (100mL) and 1,3,5-trimethoxybenzene (2-3) (16.4g, 100mmol) were added to it, stirred at 5°C After that, add H to it 2 o 2 (30%, 34g, 300mmol), stirred at 25°C for 20h, a yellow-green solid was precipitated, water (200mL) was added to the above reaction system, and 12.4g of a yellow solid was obtained by suction filtration, yield 74%, m.p.252-255°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com