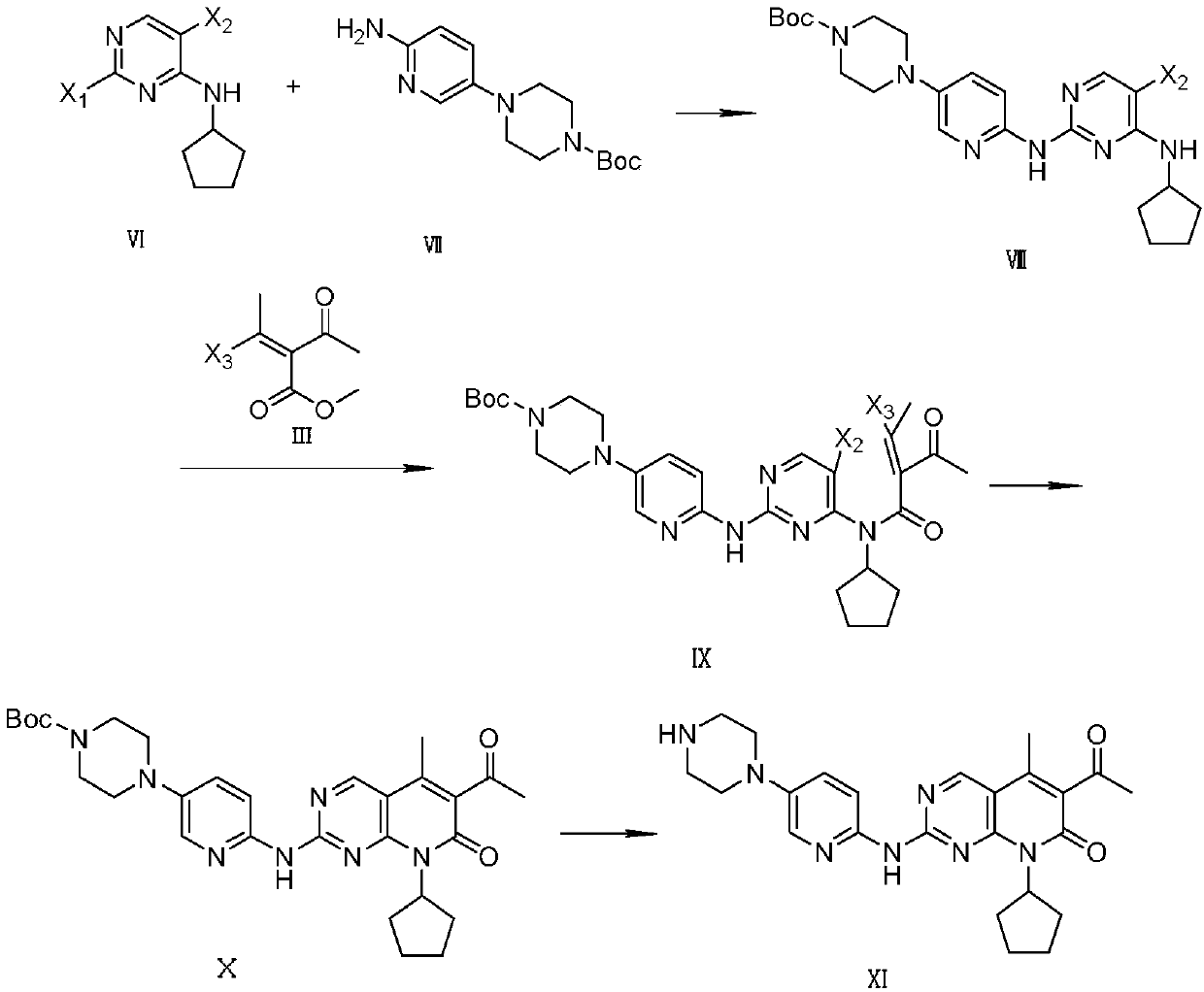
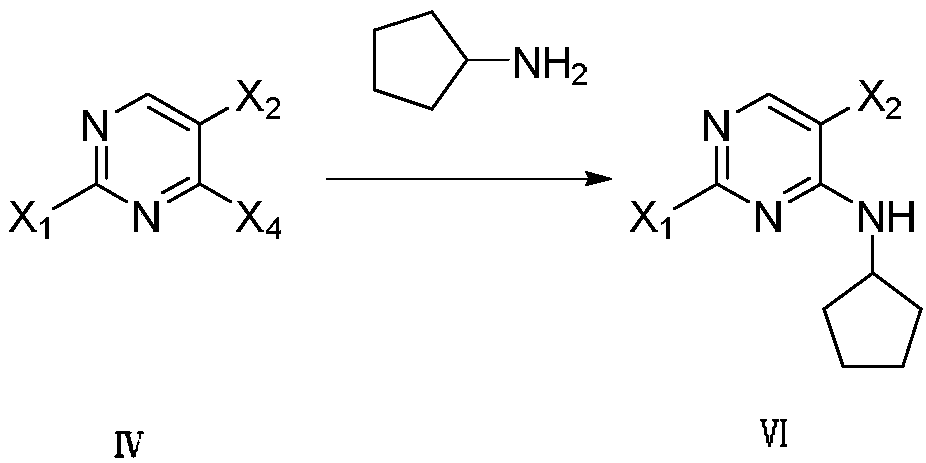
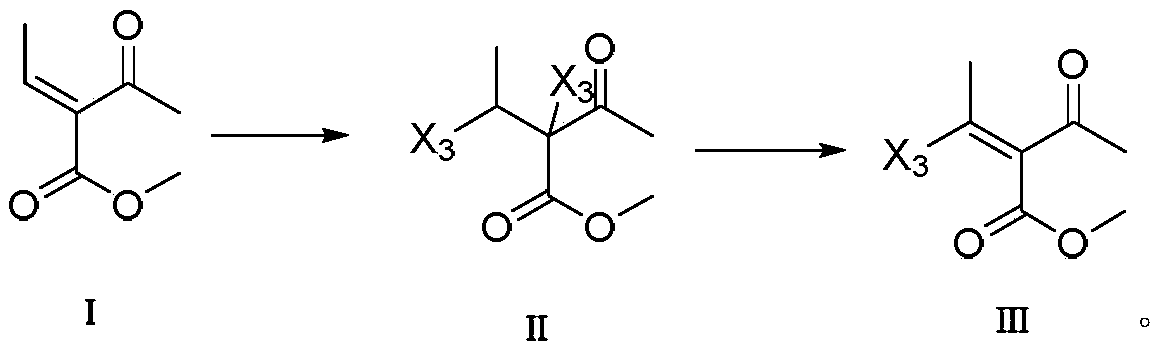
Method for synthesizing palbociclib
A technology of palbociclib and compounds, which is applied in the field of drug synthesis, can solve the problems of low product yield and high production cost, achieve good reaction selectivity, reduce production cost, and be suitable for industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] The preparation of embodiment 1,2-acetyl-2,3-dibromobutyric acid methyl ester
[0051] Add 2-acetyl-2-butenoic acid methyl ester (I) (8.52g, 60mmol) and 40mL of dichloromethane in the reaction flask, control the temperature of the reaction flask and add liquid bromine (9.9g, 62mmol) dropwise at 5°C , the dropwise addition was completed, and the reaction temperature was raised to room temperature and stirred for 30 minutes. TLC detects that the reaction is complete. Dry over anhydrous sodium sulfate, distill under reduced pressure to recover the solvent, recrystallize the obtained concentrated solid with ethyl acetate and n-hexane (1:1, V / V), and dry in vacuo to obtain 2-acetyl-2,3-dibromobutane Acid methyl ester (II-1) 17.81 g; yield 99%; purity 99.6% (HPLC area normalization method); mass spectrum (ESI): m / z 300.9 (M+H).
Embodiment 2
[0052] The preparation of embodiment 2,2-acetyl-3-bromo-2-butenoic acid methyl ester
[0053] Add 2-acetyl-2,3-dibromobutanoic acid methyl ester (II-1) (6.04g, 20mmol) and 40mL of methanol into the reaction flask, add sodium methoxide (2.7g, 50mmol) dropwise in 15mL of methanol at room temperature After the solution was dropped, the reaction temperature was kept at 40° C. and stirred for 1 hour. TLC detects that the reaction is complete. Wash 2-3 times with 4% dilute hydrochloric acid aqueous solution to make the reaction solution neutral. The organic phase was dried with anhydrous sodium sulfate, and the solvent was recovered by distillation under reduced pressure. The resulting concentrated solid was recrystallized with ethyl acetate and n-hexane (1:2, V / V), and dried in vacuo to obtain 2-acetyl-3-bromo- 2-butenoic acid methyl ester (III-1) 3.977g; yield 90%; purity 99.8% (HPLC area normalization method); mass spectrum (ESI): m / z 221.98 (M+H).
Embodiment 3
[0054] The preparation of embodiment 3,2-chloro-4-cyclopentylamino-5-bromopyrimidine
[0055] Add 5-bromo-2,4-dichloropyrimidine (IV-1) (11.4g, 50mmol), triethylamine (2.24g, 20mmol) and dichloromethane (150mL) into the reaction flask, stir well and cool to At 0-5°C, cyclopentylamine (V) (4.257g, 50mmol) was slowly added dropwise, and after the dropwise addition, the temperature was raised to 45°C to react for 6 hours, and the reaction was detected by TLC. Add 150 mL of water to quench the reaction, wash the organic phase twice with saturated brine, extract the aqueous phase twice with ethyl acetate, combine the organic phases, dry over anhydrous sodium sulfate, and recover the solvent by distillation under reduced pressure. The compound 2-chloro-4-cyclopentylamino-5-bromopyrimidine (VI-1) was separated by column chromatography with ethyl acetate mixed solvent 11.0g; the yield was 80%; the purity was 99.8% (HPLC area normalization method) ; Mass spectrum (ESI): m / z = 275.98 (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com