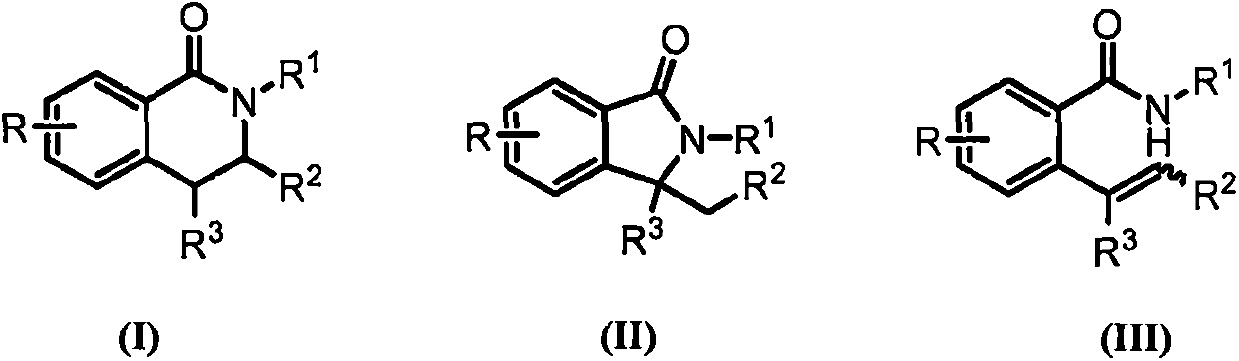
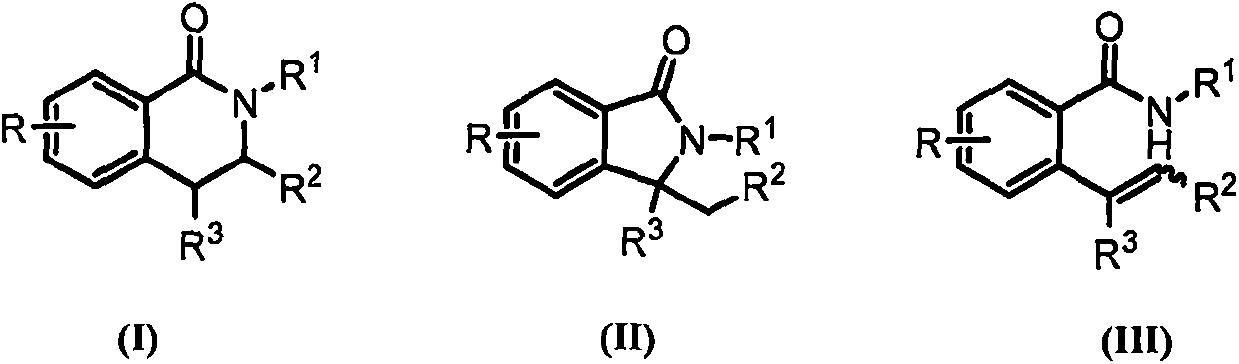
Novel synthetic method of 3,4-dihydroisoquinolin-1-one and isoindolin-1-one derivatives
A technology of dihydroisoquinoline and synthesis method, which is applied in 3 fields, can solve problems such as unstable reactants, harsh reaction conditions, and complicated preparation process, and achieve the effects of high reaction yield, simple operation, and high application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024]
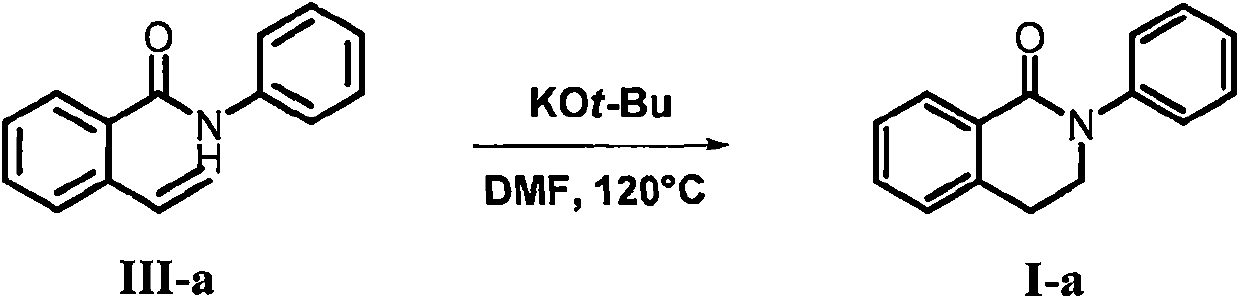
[0025] In a 250mL round-bottomed flask, add potassium tert-butoxide (0.336g, 3.0mmol), III-a (2.233g, 10.0mmol), N,N-dimethylformamide (70.0mL), under nitrogen protection at 120°C The reaction was carried out for 20 hours. After the reaction was completed, water (100 mL) was added, extracted with ethyl acetate (100 mL×3), the combined organic phase was concentrated under reduced pressure to remove the solvent, and the residue was passed through a silica gel column layer with a mixed solvent of petroleum ether and ethyl acetate as eluent. Analysis, separation and purification gave white solid I-a (2.010g, yield 90%); 1 HNMR (400MHz, CDCl 3 ): δ8.16(dd, J=7.8, 1.4Hz, 1H), 7.52-7.34(m, 6H), 7.32-7.21(m, 2H), 4.00(t, J=6.4Hz, 2H), 3.15( t, J = 6.4Hz, 2H); 13 C NMR (100MHz, CDCl 3 ): δ164.22, 143.11, 138.31, 132.06, 129.72, 128.94, 128.77, 127.22, 126.97, 126.27, 125.33, 49.43, 28.65.
Embodiment 2
[0027] Using the same method as in Example 1, sodium methoxide was used instead of potassium tert-butoxide to obtain white solid I-a (1.608 g, yield 72%).
Embodiment 3
[0029] Using the same method as in Example 1, using dimethyl sulfoxide instead of N,N-dimethylformamide as the solvent, a white solid I-a (1.786 g, yield 80%) was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com