Sulfonium compound, resist composition, and patterning process
一种锍化合物、抗蚀剂的技术,应用在有机化学、图纹面的照相制版工艺、仪器等方向,能够解决含氟起始反应物昂贵、环境安全不能令人满意、不足以精确控制酸扩散等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
[0311] Embodiment 1-1: Synthesis of Intermediate A
[0312]
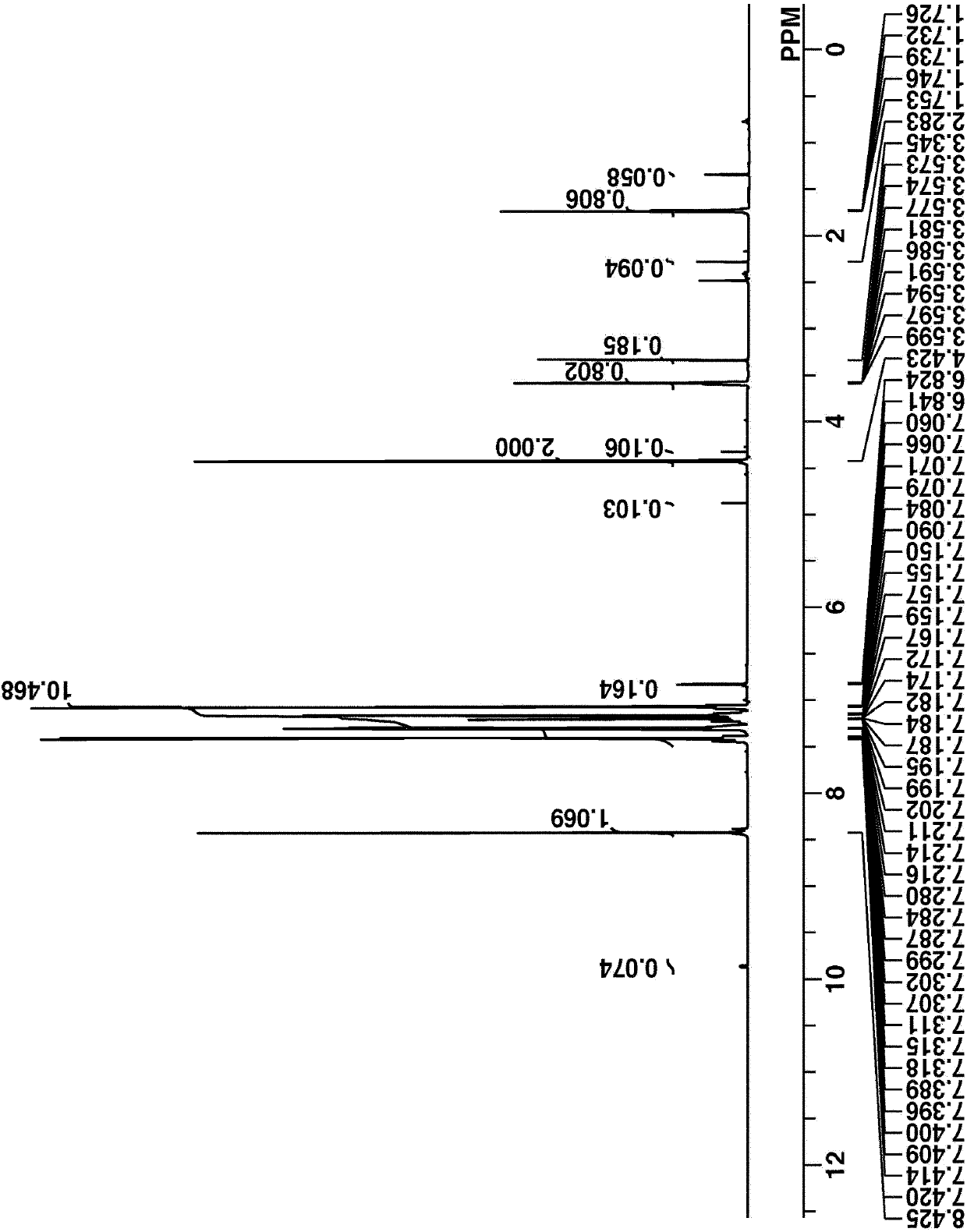
[0313] 220 g of 4-phenylthiophenol were dissolved in a mixture of 550 g THF and 133 g water. At room temperature, 25% by weight of caustic soda was added dropwise to the solution aged for 15 minutes. Thereafter, a solution of 352 g of Compound A dissolved in 250 g of THF was added dropwise thereto at room temperature. The solution was aged overnight, and then 5% by weight hydrochloric acid was added thereto for quenching. The reaction solution was diluted with 620 g of hexane and 620 g of toluene, and washed with water. 1% by weight of caustic soda was added to the organic layer, followed by separation. The organic layer was mixed with 2.5% by weight hydrochloric acid, washed with water again, and then separated. The organic layer was concentrated to obtain 208 g of the final compound, Intermediate A, as a colorless oil (yield 65%).
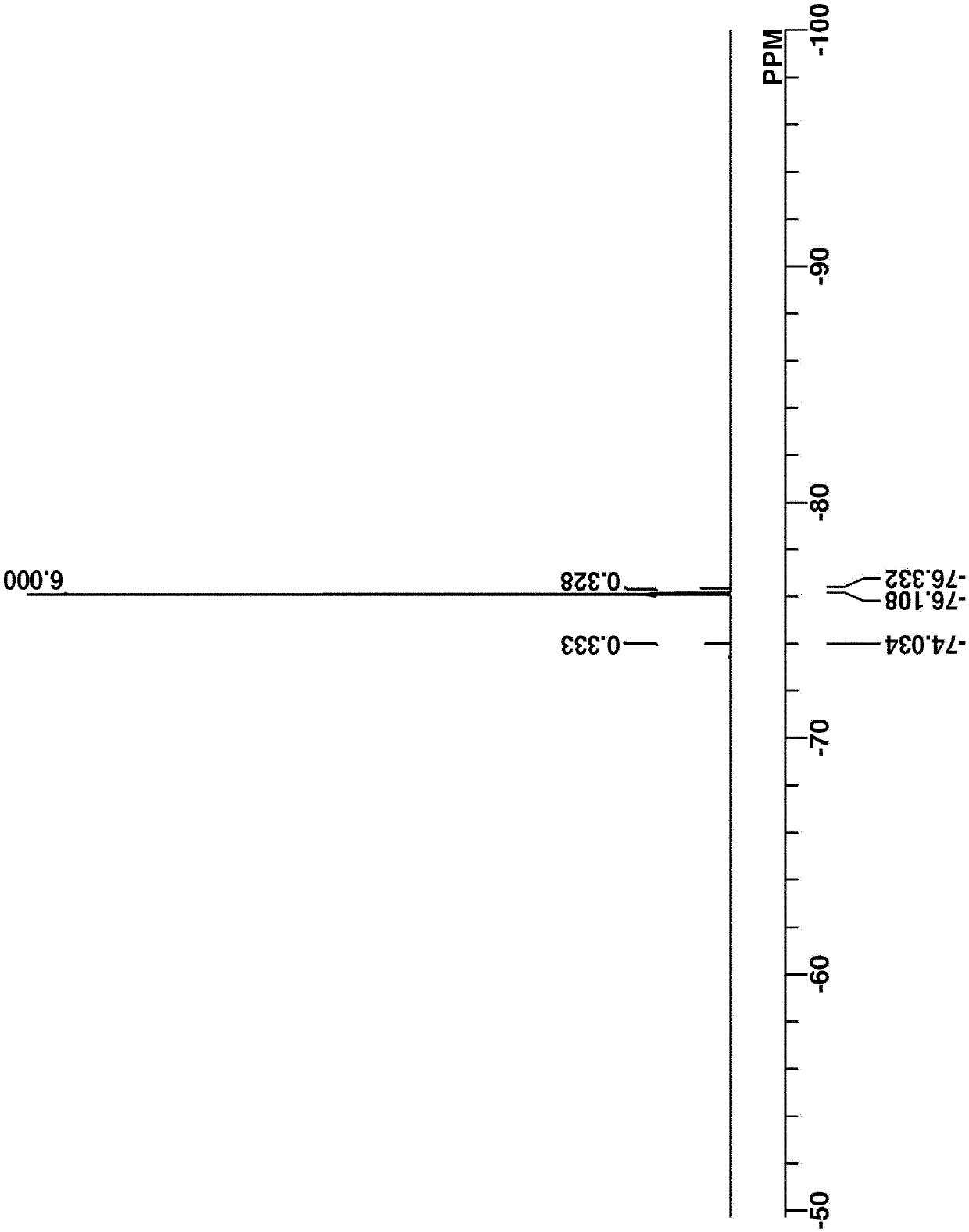
[0314] Intermediate A was analyzed by spectroscopy. figure 1 and 2 The...
Embodiment 1-2
[0317] Embodiment 1-2: the synthesis of intermediate B
[0318]
[0319] 183 g of Intermediate A were dissolved in 1300 g of acetic acid. Under ice cooling, 51 g of 35% by weight aqueous hydrogen peroxide was added to the solution. The solution was matured overnight at room temperature, and then a solution of 25 g of sodium thiosulfate dissolved in 120 g of water was added dropwise at room temperature. After stirring for 1 hour, the reaction solution was diluted with 2000 g of ethyl acetate and 1000 g of toluene, and washed with 1000 g of water. The organic layer was mixed with 1% by weight caustic soda, then separated. The organic layer was washed once with water, mixed with 2.5% by weight hydrochloric acid, and then separated. The organic layer was washed with water and concentrated, and ethyl acetate was added to the concentrate to form a 50% by weight ethyl acetate solution. The solution was added dropwise to a mixture of n-hexane and toluene (weight ratio 2:1) fo...
Embodiment 1-3
[0323] Example 1-3: Synthesis of Intermediate C
[0324]
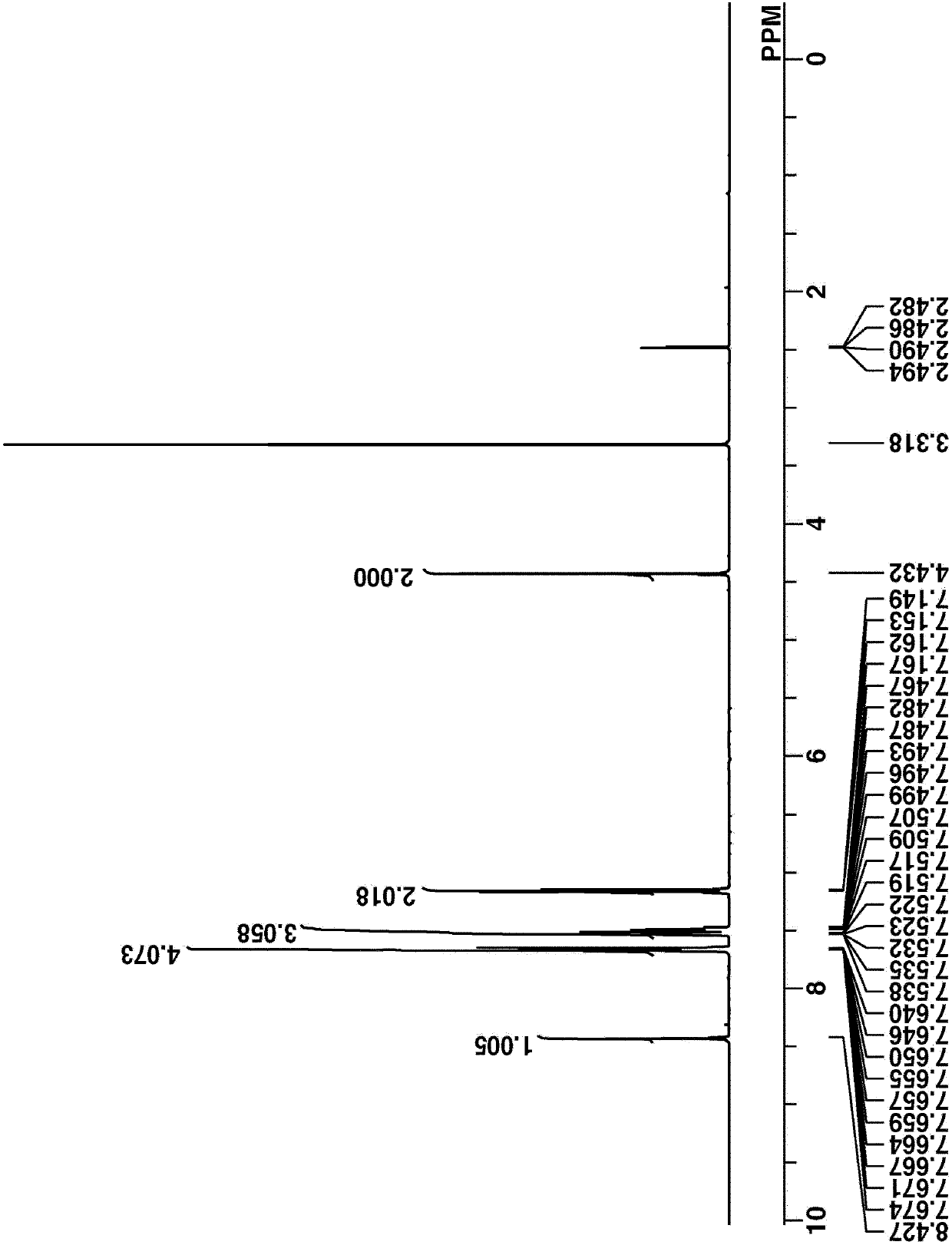
[0325] 117 g of Intermediate B were dissolved in a mixture of 69 g of diisopropylethylamine and 590 g of acetonitrile. Under ice-cooling, 36 g of chloromethyl methyl ether was added dropwise to the solution. The solution was aged overnight at room temperature, then mixed with 800 g of water and 800 g of toluene, and then separated. The organic layer was washed once with water, washed with 1% by weight ammonia, and then washed with water again. The solution was further washed with 1% by weight hydrochloric acid, and washed with water. The organic layer was taken out and concentrated under reduced pressure. The obtained solid was dried in vacuo to obtain 127 g of the final compound, Intermediate C, as white crystals (yield 82%).
[0326] Intermediate C was analyzed by spectroscopy. Figure 5 and 6 The NMR spectrum is shown in DMSO-d 6 of 1 H-NMR and 19 F NMR. exist 1 In H-NMR analysis, a small amount of r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| refractive index | aaaaa | aaaaa |
| weight-average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com