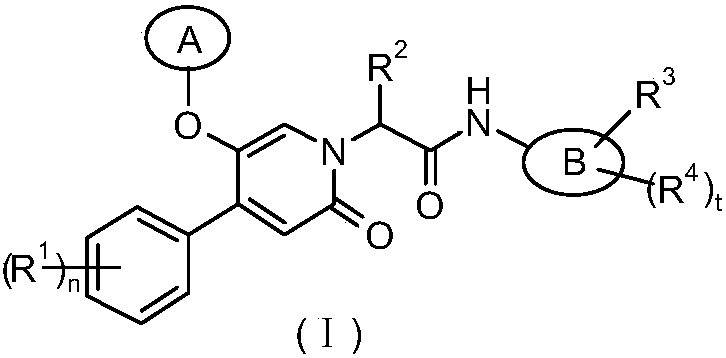
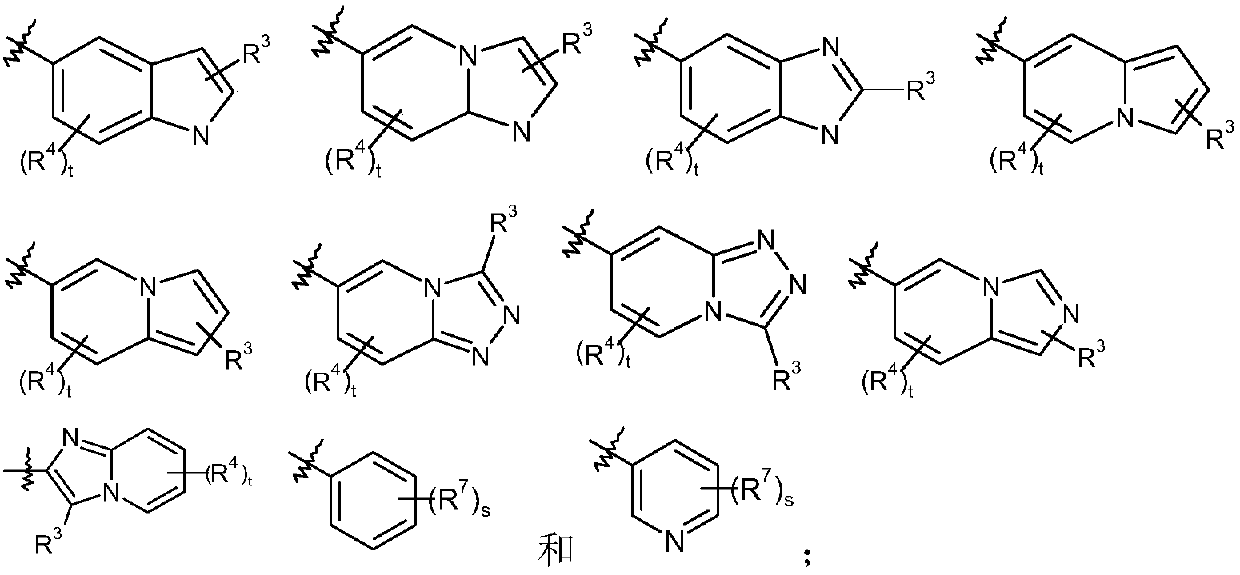
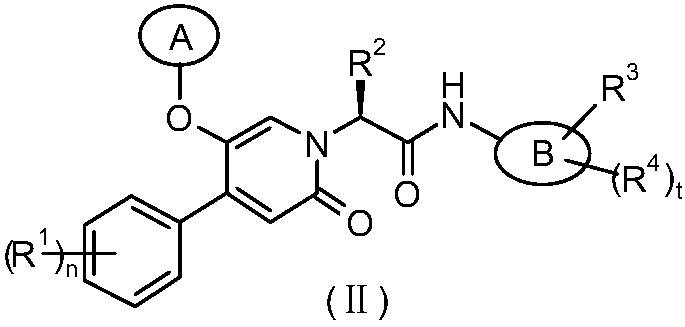
Epoxy-substituted oxopyridine derivatives and their preparation method and application in medicine
A technology of heterocyclic group and cycloalkyl group, which is applied in the field of epoxy-substituted oxopyridine derivatives, its preparation and its application in medicine, and can solve the problems of negligible effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0159] 5-(2-(4-(5-chloro-2-benzonitrile)-5-(cyclopentyloxy)-2-oxopyridin-1(2H)-yl)-4-methoxybutyrylamino )-1H-indole-2-carboxylic acid
[0160]
[0161] first step
[0162] Methyl 2-bromo-4-methoxybutyrate 1b
[0163] Add methyl 4-methoxybutyrate 1a (1.6g, 12.1mmol) into 50mL of tetrahydrofuran, cool down to -78°C with a dry ice-acetone bath, and slowly add lithium bistrimethylsilylamide (12.7mL, 12.7mmol), added, stirred and reacted for 1 hour, added trimethylchlorosilane (1.31g, 12.1mmol), continued to stir and reacted for 20 minutes, then added N-bromosuccinimide (2.15g, 12.1mmol) , and stirred for 2 hours. The dry ice-acetone bath was removed, the temperature of the reaction solution was naturally raised to room temperature, the reaction was quenched with saturated ammonium chloride solution, the reaction solution was extracted with ethyl acetate (50mL×3), the organic phases were combined, and the organic phases were respectively saturated with water (50mL). Washed ...
Embodiment 2
[0196] 5-(2-(4-(5-chloro-2-benzonitrile)-5-cyclobutyloxy-2-oxopyridin-1(2H)-yl)-4-methoxybutyramide
[0197] base)-1H-indole-2-carboxylic acid
[0198]
[0199] first step
[0200] 5-cyclobutoxy-2-methoxypyridine 2b
[0201] Dissolve 1c (5.0g, 39.96mmol) in 50mL of N,N-dimethylformamide, add potassium carbonate (11.05g, 79.92mmol), heat to 95°C, add cyclobutyl bromide 2a (6.20g , 45.95mmol), the addition was completed, and the reaction solution was stirred at 90°C for 16 hours. Heating was stopped, the temperature of the reaction solution was naturally cooled to room temperature, 15 mL of water was added to the reaction solution, extracted with ethyl acetate (20 mL × 3), the organic phases were combined, and the organic phase was washed with a saturated sodium chloride solution (50 mL × 3). Dry over sodium sulfate, remove the desiccant by filtration, concentrate the filtrate under reduced pressure, and purify the resulting residue by silica gel column chromatography with...
Embodiment 3
[0229]5-((S)-2-(4-(5-Chloro-2-benzonitrile)-2-oxo-5-((R)-tetrahydrofuran-3-oxo)pyridin-1(2H)-yl) -3-phenylpropanamide)-1H-indole-2-carboxylic acid 3
[0230]
[0231] first step
[0232] (R)-2-methoxy-5-(tetrahydrofuran-3-oxo)pyridine 3b
[0233] 1c (1.78g, 14.24mmol) and (S)-tetrahydrofuran-3-yl-4-methylbenzenesulfonyl ester 3a (3.45g, 14.24mmol) were sequentially prepared using a known method "Guangzhou Chemical Industry, 2012, 40 (16 ), 62-63” prepared), dissolved in 20mL of N,N-dimethylformamide, added potassium carbonate (4.92g, 35.60mmol), heated to 95°C, and the reaction solution was stirred at 90°C for 16 Hour. Heating was stopped, the temperature of the reaction solution was naturally cooled to room temperature, 50 mL of water was added to the reaction solution, extracted with ethyl acetate (40 mL × 3), the organic phases were combined, and the organic phase was washed with a saturated sodium chloride solution (25 mL × 3). Dry over sodium sulfate, remove the de...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com