Derivatization and separation analysis method of amino pyrazolone iso-bifunctional reagents of reducible carbohydrate chains and glycoprotein O-carbohydrate chains
A technology of aminopyrazolone and two functional groups, applied in the field of glycobiology, can solve the problems of low derivatization efficiency, inability to realize further analysis of sugar chains, harsh reaction conditions, etc., achieve short reaction time, strong versatility, The effect of high derivatization efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment
[0094] The examples in the present invention are about the derivatization method of aminopyrazolone heterobifunctional reagents of reducing sugar chains, and the preliminary purification, separation and identification methods of derivatized products. The examples in the present invention also describe the derivatization method of aminopyrazolone-based heterobifunctional reagents of glycoprotein O-sugar chains.
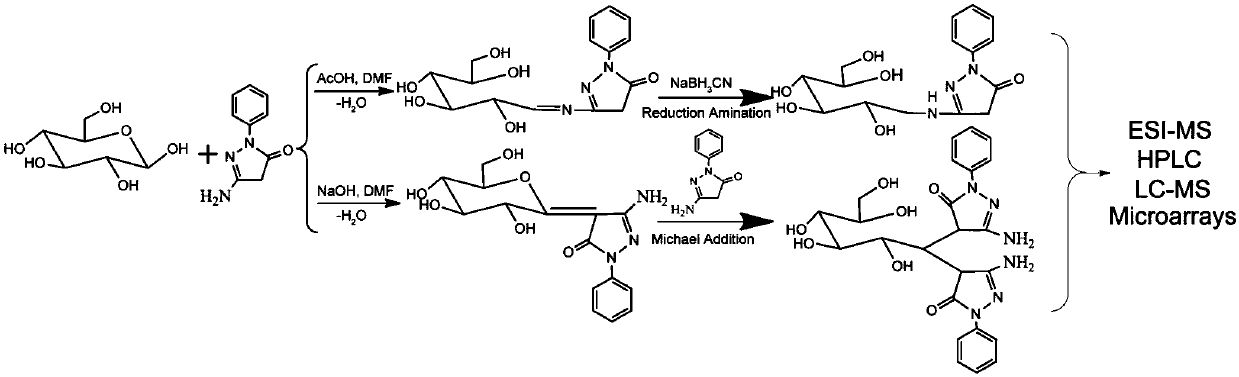
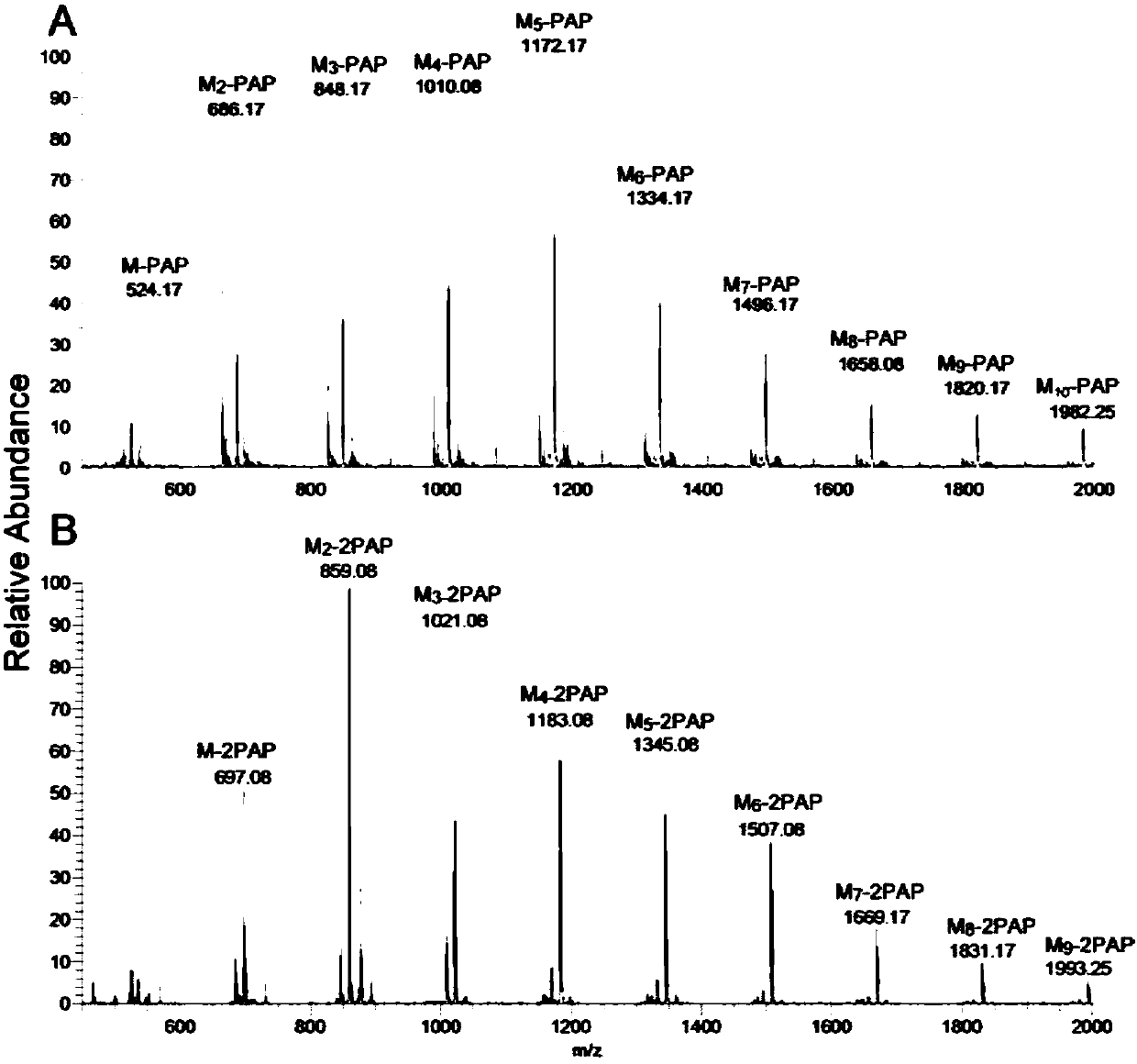
[0095] Taking PAP as an example, the process of two test reactions for the derivatization of reducing sugar chains by aminopyrazolone heterobifunctional reagents is as follows: figure 1 shown. First, for the free reducing sugar chains, taking maltodextrin-oligosaccharide mixture as an example, derivatize through two different reaction modes. First, under acidic conditions, the sugar chains are first dissolved in PAP / DMF and a weak acid system for reaction, and then NaBH is added to the above system 3 After the CN reaction is completed, after purification, the active ...
Embodiment 1
[0098] Example 1: Derivatization of maltodextrin (Maltodextrin) under acidic conditions and preliminary purification and detection of derivatives
[0099] (1) Derivatization
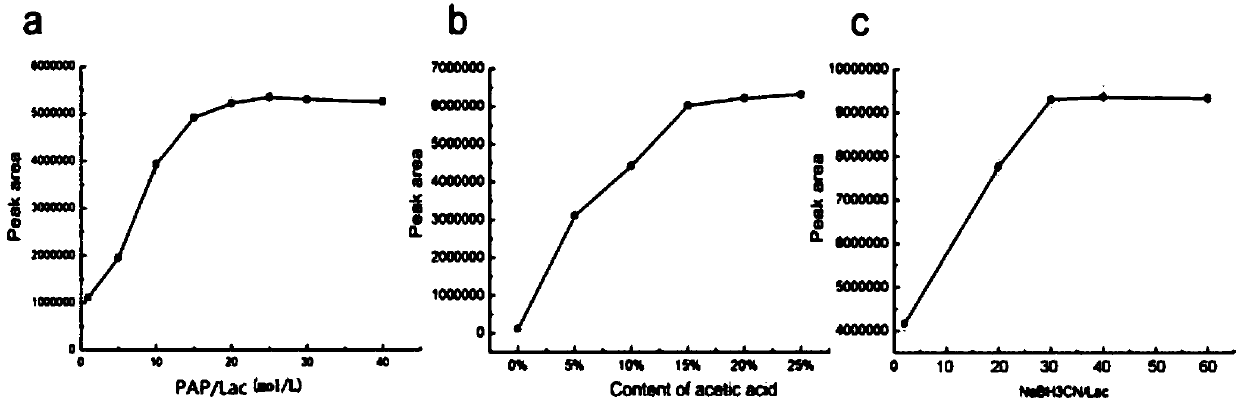
[0100] Dissolve PAP in DMF to prepare a solution with a concentration of 0.625 mol / L, weigh 5 mg of maltodextrin (Maltodextrin (Maltodextrin) is a macromolecular reducing oligosaccharide) and add it to 500 μL of PAP solution, and then add to the reaction 150 μL of glacial acetic acid was added to the system, and the reaction mixture was reacted at 70° C. for 2 hours. After the reaction is over, add NaBH with a concentration of 0.75mol / L to the mixture 3 The aqueous solution of CN was 500 μL, and the obtained reaction system was continued to react at 70° C. for 1 hour. After the reaction, cool to room temperature, wash with dichloromethane three times, 1.5mL-2mL each time, and collect the crude product after the aqueous phase is concentrated.
[0101] (2) The crude product of the derivative is prelimin...
Embodiment 2
[0118] Example 2: Derivatization of maltodextrin (Maltodextrin) under alkaline conditions and preliminary purification and detection of derivatives
[0119] (1) Derivatization
[0120] Dissolve PAP in DMF to prepare a solution with a concentration of 0.625 mol / L, weigh 5 mg of maltodextrin (maltodextrin is a macromolecular reducing oligosaccharide) and add it to 500 μL of PAP solution, and then add to the reaction system 500 μL of 0.4 mol / L sodium hydroxide aqueous solution was added, and the resulting reaction system was reacted at 50° C. for 1.5 h. After the reaction, cool to room temperature, adjust the pH to 7 with 1M hydrochloric acid aqueous solution, then wash once with dichloromethane, collect the aqueous phase and add 3-5 drops of glacial acetic acid, and then wash with 1.5mL-2mL dichloromethane. The aqueous phase was collected, concentrated under reduced pressure, dried, dissolved in water, and washed once with dichloromethane to obtain a crude product of the deriva...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com