Chemical lithiation of electrode active material
An electrode active material, electrochemical technology, used in electrochemical generators, negative electrodes, electrode manufacturing and other directions, can solve problems such as problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0086] Under an argon atmosphere, 50 g of polycrystalline silicon were added with stirring to 500 ml of a 2M solution of phenyllithium in cyclohexane / diethyl ether (70 / 30). The resulting suspension was stirred at room temperature for 10 hours. The prelithiated silicon was then filtered off and washed with cyclohexane / diethyl ether (70 / 30). 7 g of pre-lithiated silicon was treated with 2 g of carbon black, SBR in 1 g of toluene to give a paste. It was applied on both surfaces of the current collector 31 made of copper foil. The solvent was removed under reduced pressure. The electrode thus prepared can be used as the negative electrode 21 in a lithium ion battery.
Embodiment 2
[0088] with H 2 O treated a mixture of 7 g of silicon, 2 g of carbon black, and 1 g of polyacrylic acid (PAA) to obtain a paste. It was applied on both surfaces of the current collector 31 made of copper. The solvent was removed under reduced pressure. The electrode thus prepared was placed in a hexane solution (1.5 mol / L) of n-hexyllithium at room temperature for 30 minutes under an argon atmosphere. The pre-lithiated electrode is then washed with hexane and, after removing any solvent residue, can be used as the negative electrode 21 in a Li-ion battery.
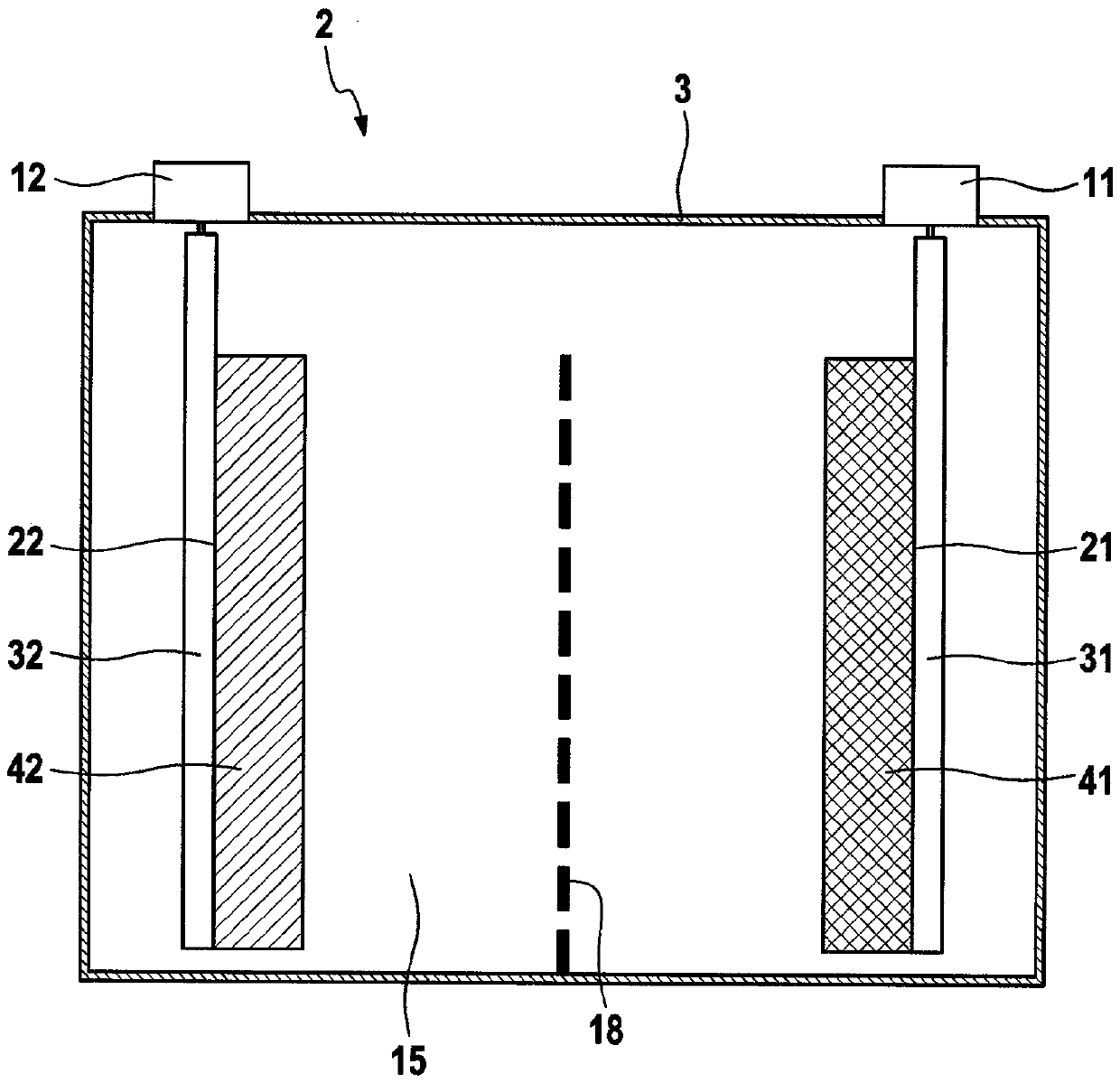
[0089] exist figure 1 An electrochemical energy storage system 2 in the form of a battery cell is schematically shown in . The energy storage system 2 contains a battery housing 3 which is designed in the shape of a prism, here cuboid. The battery housing 3 is here electrically conductive and is made, for example, of aluminum. The battery housing 3 can however also be made of an electrically insulating material, for ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com