Electrochemical method for catalytic oxidation of methanol with polygonal PtCoFe nano-catalyst
A nano-catalyst and catalytic oxidation technology, which is applied in electrochemical generators, nanotechnology for materials and surface science, nanotechnology, etc., can solve the problems of low catalytic activity, high price, and short life of catalysts, and achieve wide application Prospect, effect of high active site density and excellent electrocatalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
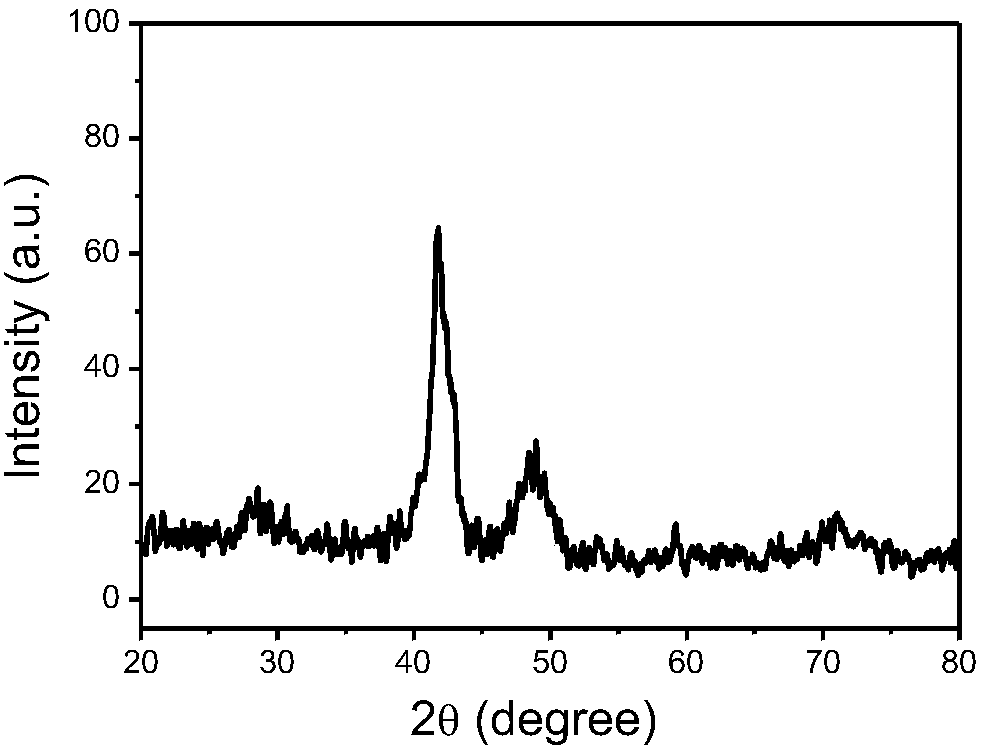
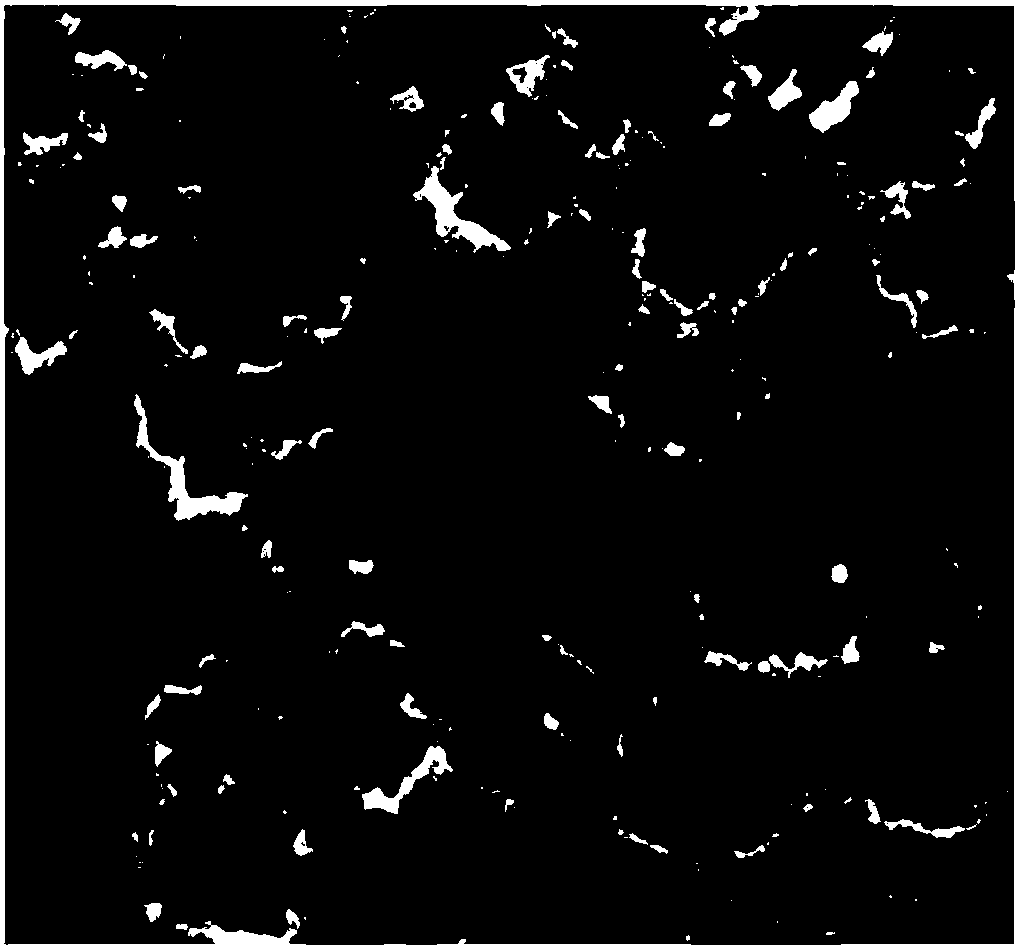
[0022] Measure 1.0mL chloroplatinic acid (19.3mmol / L), 4.0mL concentration is the cobalt chloride of 1.66mmol / L and 3.0mL concentration is the iron trichloride aqueous solution of 1.66mmol / L in the 30ml reactor, then add poly Vinylpyrrolidone K30, hexadecyltrimethylammonium bromide CTAB and NaBr are stirred and dissolved with a magnetic stirrer, and then the air in the reactor is exhausted with hydrogen, and 0.8MPa hydrogen is introduced into the reactor, and then heated at 200°C The reaction is carried out under heating, and after the reaction is completed, through ethanol centrifugal washing, freeze-drying and other processing steps to obtain polygonal PtCoFe alloy nanoparticles (such as figure 2 Shown), wherein, the consumption scope of polyvinylpyrrolidone K30 is 210mg, and the consumption scope of cetyltrimethylammonium bromide is 60mg, and the consumption of NaBr is identical with the consumption of cetyltrimethylammonium bromide.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com