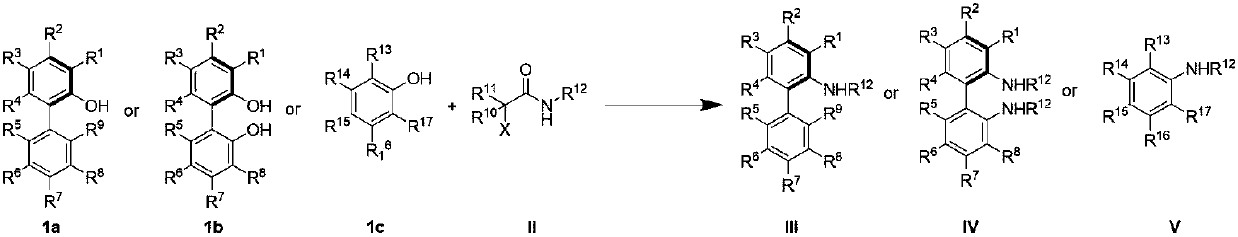
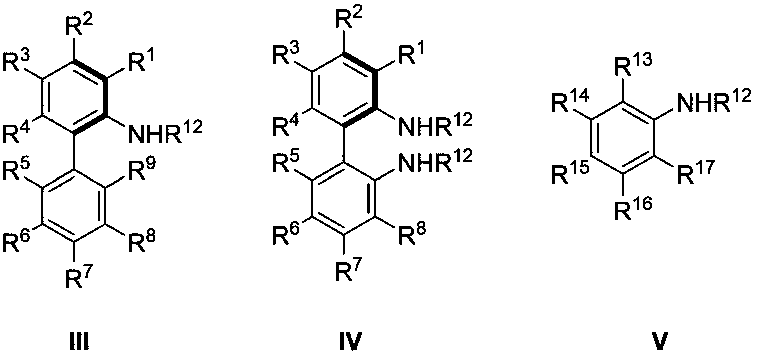
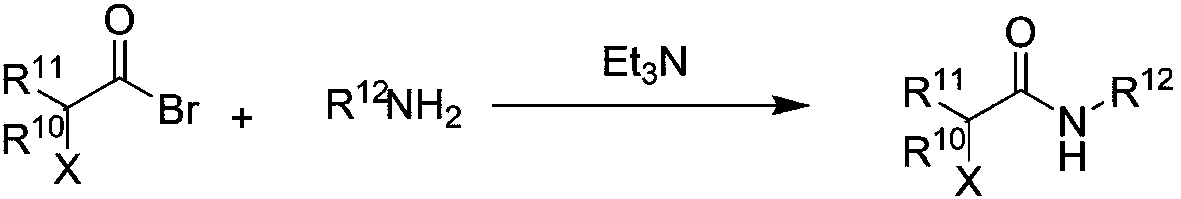Chiral aromatic amine compounds and preparation method thereof
A technology of amine compounds and aromatic amines, applied in the field of chiral aromatic amine compounds and their preparation, can solve the problems of uneconomical and environmental protection, high toxicity of β-naphthylamine, and large environmental impact
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1: Preparation of (R)-2'-amino-[1,1'-binaphth]-2-ol (3a)
[0023]
[0024] Under air environment, add 1a (0.2mmol), 2e (0.3mmol), potassium carbonate (0.3mmol), potassium iodide (0.02mmol), dimethyl sulfoxide (2ml) as reaction solvent in reaction tube, in reaction tube After adding a rubber stopper, put it into an oil bath heated to 50°C in advance and stir for 24 hours. Thin-layer chromatography (TLC) spots the plate to determine the end point of the reaction. When the TLC plate shows that the reaction raw material 1a disappears completely, the reaction tube is lifted from the oil bath, and potassium hydroxide (2.0mmol) is directly added to the reaction tube, and then put into The temperature was raised to 150°C in an oil bath and stirred for 4 hours. After the reaction was completed, the reaction tube was cooled to room temperature, and 5 ml of water was added to quench the reaction, then extracted with ethyl acetate and spin-dried, and separated by column ...
Embodiment 2
[0026] Example 2: Preparation of (R)-2'-phenyl-[1,1'-binaphthyl]-2-amine (3b)
[0027]
[0028] Under air environment, add 1b (0.2mmol), 2b (0.3mmol), potassium carbonate (0.3mmol), potassium iodide (0.02mmol), dimethyl sulfoxide (2ml) as reaction solvent in reaction tube, in reaction tube After adding a rubber stopper, put it into an oil bath heated to 50°C in advance and stir for 24 hours. Thin-layer chromatography (TLC) spots the plate to determine the end point of the reaction. When the TLC plate shows that the reaction material 1b disappears completely, the reaction tube is lifted from the oil bath, and sodium hydroxide (6mmol) is directly added to the reaction tube, and then put into the pre- The temperature was raised to 130°C and stirred in an oil bath for 4 hours. After the reaction was completed, the reaction tube was cooled to room temperature, and 5 ml of water was added to quench the reaction, then extracted with ethyl acetate and spin-dried, and separated by c...
Embodiment 3
[0030] Example 3: Preparation of (R)-2'-methyl-[1,1'-binaphthyl]-2-amine (3c)
[0031]
[0032] (R)-2'-methyl-[1,1'-binaphth]-2-ol and 2b were used as the starting substrate of the reaction to prepare the target product 3c, and the preparation method was the same as in Example 2.
[0033] Product yield 96%; yellow solid; 99% ee; 1 H NMR (400MHz, CDCl 3 )δ7.90–7.85(m,2H),7.78(d,J=8.5Hz,2H),7.52(d,J=8.4Hz,1H),7.42–7.36(m,1H),7.25–7.18(m ,3H),7.13(t,J=7.5Hz,1H),7.09(d,J=8.7Hz,1H),6.88(d,J=8.3Hz,1H),3.46(br s,2H),2.13( s,3H). 13 C NMR (101MHz, CDCl 3 )δ141.65,136.15,133.84,132.89,132.73,132.16,129.21,129.04,128.27,128.17,128.15,128.10,126.65,126.53,125.66,125.30,124.11,122.33,118.20,116.37,20.11.ATR-FTIR(cm -1 ):3475,3381,3052,2922,2853,1619,1512,1380,813,746.ESI-MS:calculated[C 21 h 17 N+H] + :284.1433,found:284.1443.[α] 20 D =+5.8 (c=0.96, CH 2 Cl 2 ).HPLC: (OD-H, hexane / i-PrOH=95 / 5, detector: 254nm, flow rate: 1mL / min), t 1 (major)=8.7min,t 2 (minor) = 10.2 min...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com