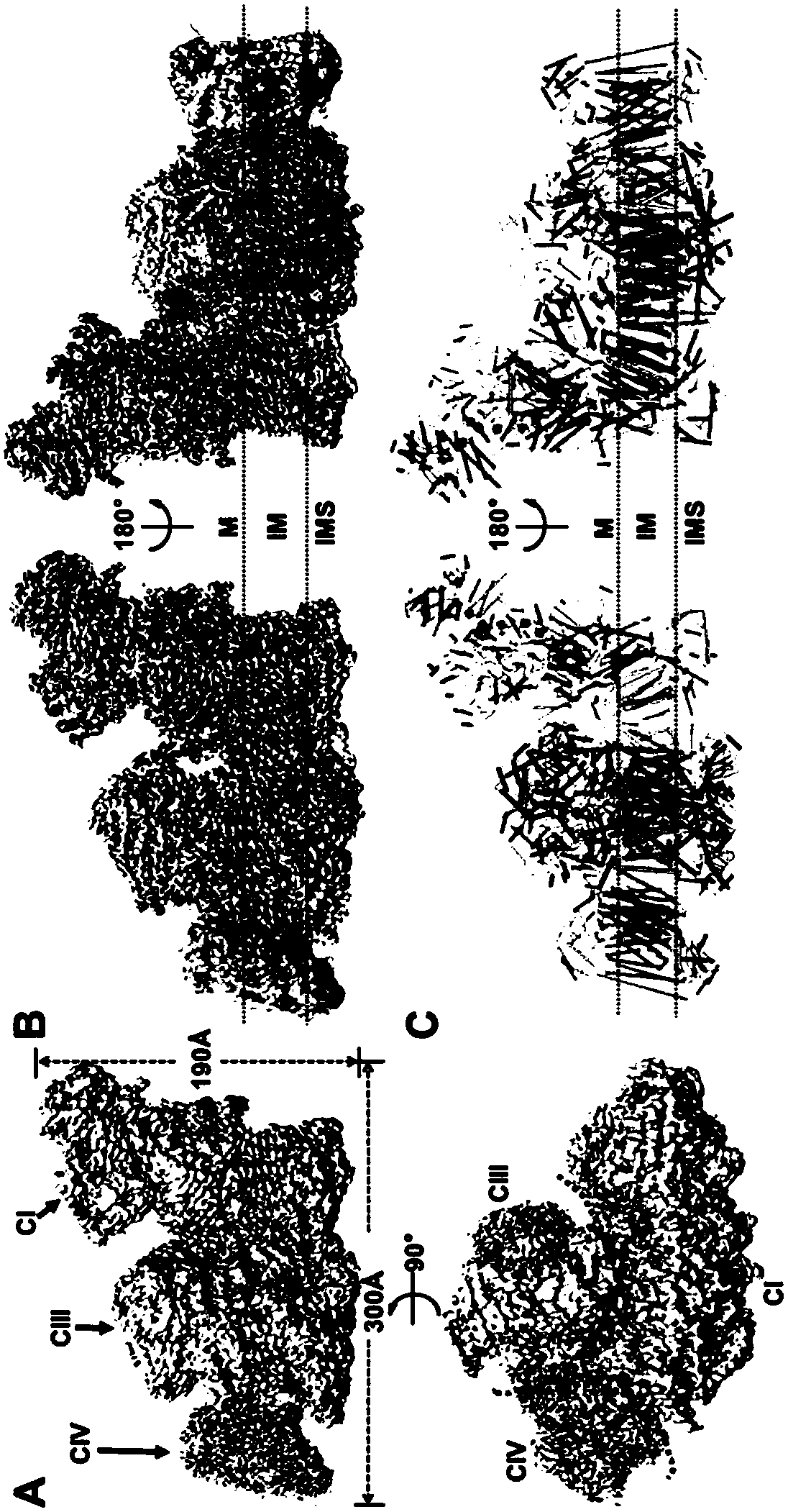
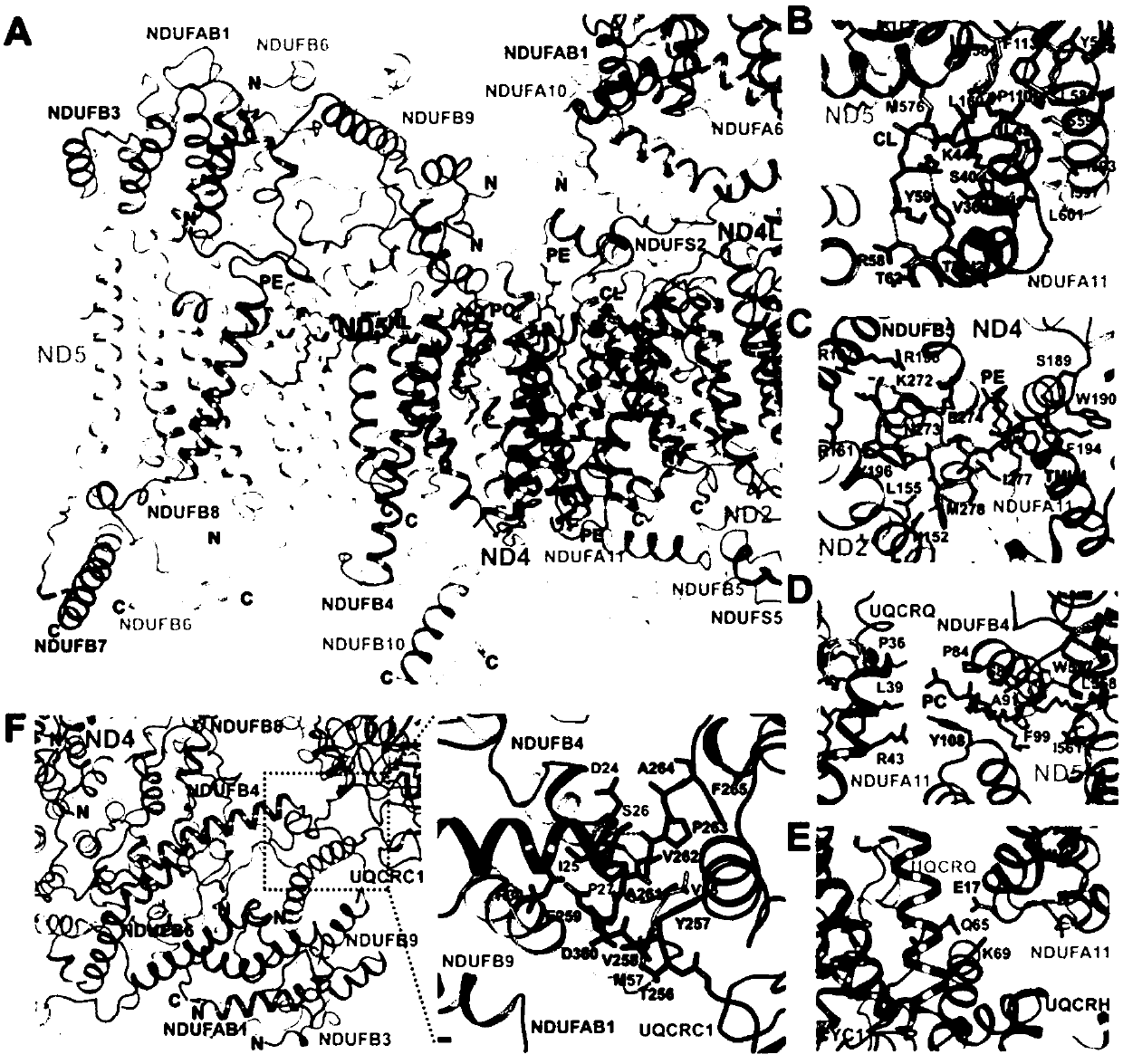
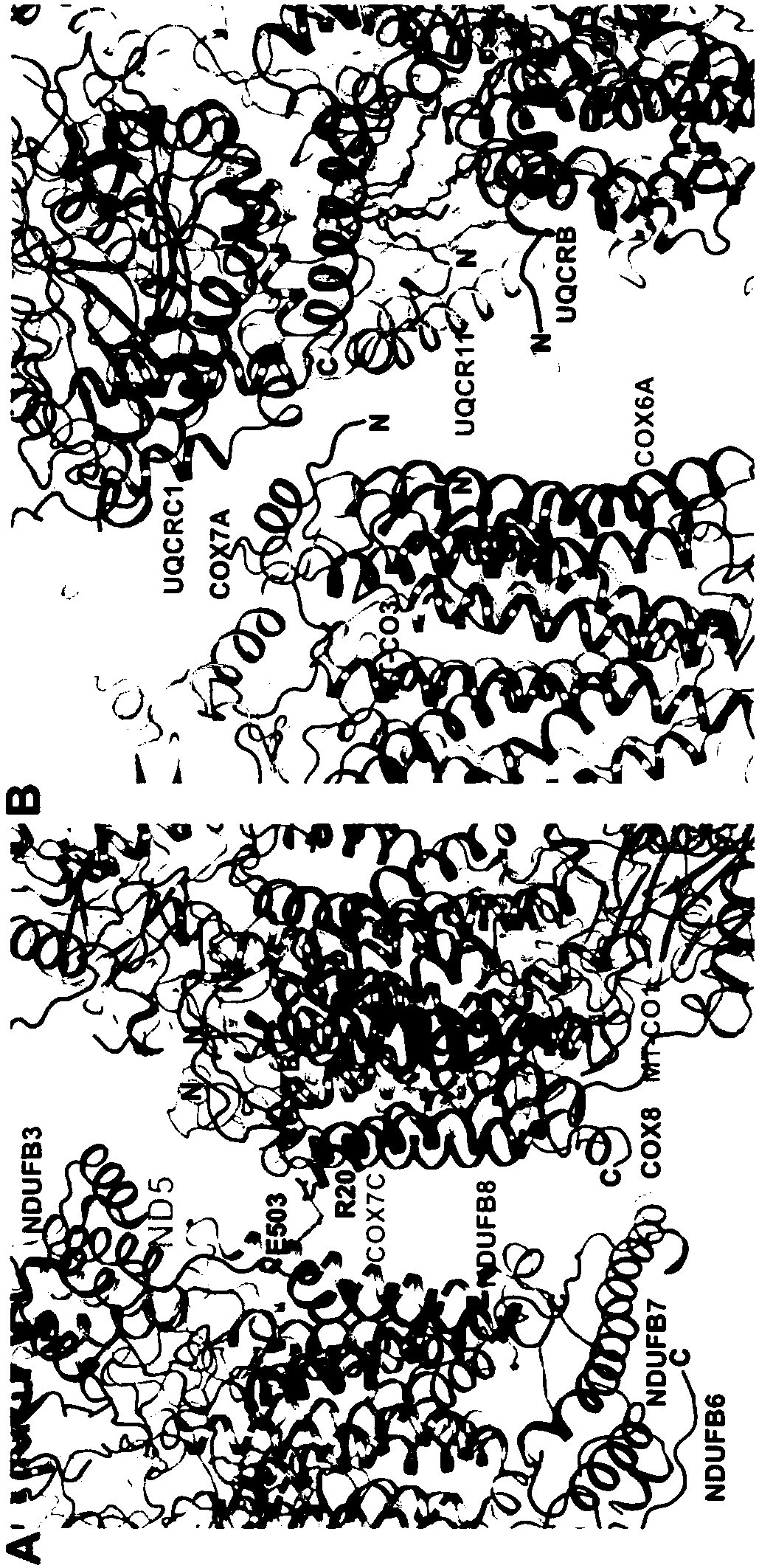
Super respiratory chain compound protein
A complex and respiratory chain technology, applied in the field of mammalian cell respiratory chain super complex protein, can solve the problem of unclear mechanism of action of timosaponin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0124] Example 1. Respiratory Chain Supercomplex Protein I 1 III 2 IV 1 purification of
[0125] Extraction of mitochondria
[0126] 100g of fresh porcine heart tissue was cut into 2cm×2cm×2cm pieces and suspended with 100mL buffer A (100mM Tris pH7.4, 225mM sorbitol, 60mM KCl, 1mM EGTA and 0.1% bovine serum albumin). Use a large-capacity tissue homogenizer to crush for 300 seconds. Transfer the homogenate to a 50mL centrifuge tube, centrifuge at 3000g for 10 minutes (min), discard cell debris and other precipitates, and retain the supernatant. Transfer the supernatant to a high-speed centrifuge tube and centrifuge at 20,000 for 30 minutes to obtain a precipitate, which is the crudely extracted mitochondria. Prepare buffer B (100mM Tris pH 7.4, 250mM sucrose, 60mM KCl, 40% Percoll (silica particle suspension treated with polyvinylpyrrolidone) and 0.1mM EGTA), resuspend the crudely extracted mitochondria with 100mL buffer B, Centrifuge at 60,000 for 50 minutes, and then...
Embodiment 2
[0137] Example 2. Respiratory Chain Supercomplex Protein I 1 III 2 IV 1 Collection of cryo-EM data and analysis of protein structure
[0138] Electron Microscopy Sample Preparation
[0139] 4 μL of the supercomplex protein soaked with small molecule drugs was dropped onto a 400-mesh Quantifoil R1.2 / 1.3 copper grid (purchased from Quantifoil, Micro Tools GmbH, Germany) covered with a carbon film in advance, and allowed to stand for 1.5 seconds. Frozen samples were then prepared using an FEI Mark IV Vitrobot sample preparation machine.
[0140] Electron Microscopy Data Acquisition
[0141] 300kVTitan Krios is used to collect high-resolution image data, and the detector is Falcon IIdirect electron detector (FEI Company). The control software for data acquisition is EPU (FEI Company). The magnification of the electron microscope is 75,000×, the pixel size is 1.08 angstroms, the defocus value is -1.3 μm to -2.3 μm, the electron dose is 35 / pixel / s, the exposure time is 1.5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com