Method for producing injection type preparation for 3D printed artificial bone
A 3D printing and injection technology, applied in the medical field, can solve the problems of unsuitable storage, a single type of slow-release drug, and the inability to use multiple drugs in combination, so as to achieve the effect of controllable dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
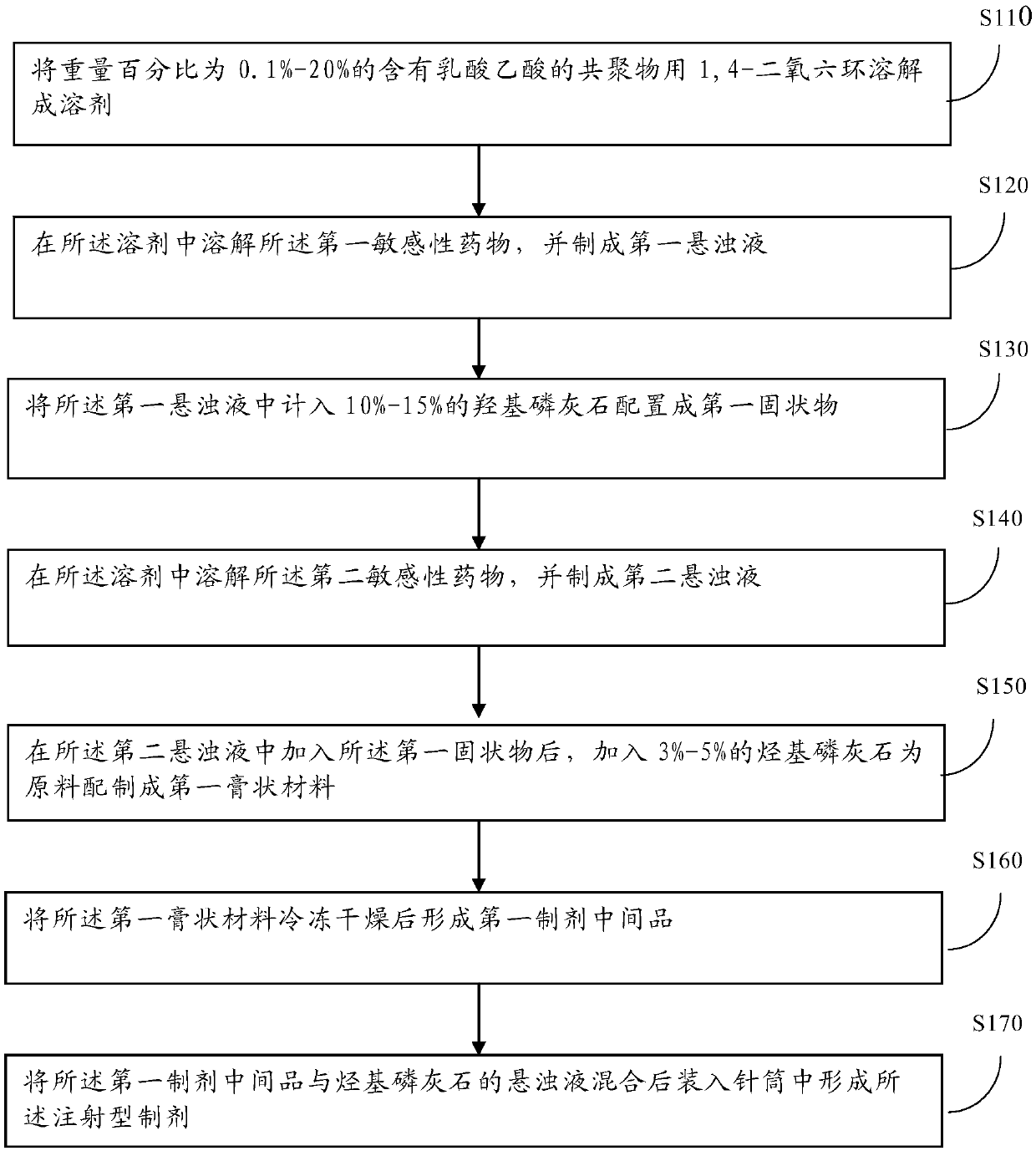
[0031] figure 1 It is a schematic flowchart of a manufacturing method of 3D printing artificial bone provided in the embodiment of the present application. Such as figure 1 As shown, the method includes:
[0032] Step 110: dissolving the 0.1%-20% by weight copolymer containing lactic acid acetic acid into a solvent with 1,4-dioxane;
[0033] Step 120: Dissolving the first sensitive drug in the solvent to make a first suspension;
[0034] Specifically, the sensitive drug refers to the drug required for long-term sustained-release treatment of the target object during the treatment of osteomyelitis. Among them, according to the level of drug sensitivity, it can be divided into the first sensitive drug, the second sensitive drug, and the third sensitive drug, wherein, according to the degree of sensitivity to the target object, it can be defined as: the first sensitive drug The sensitivity is lower than the second sensitive drug, and the sensitivity of the second sensitive dr...
Embodiment 2
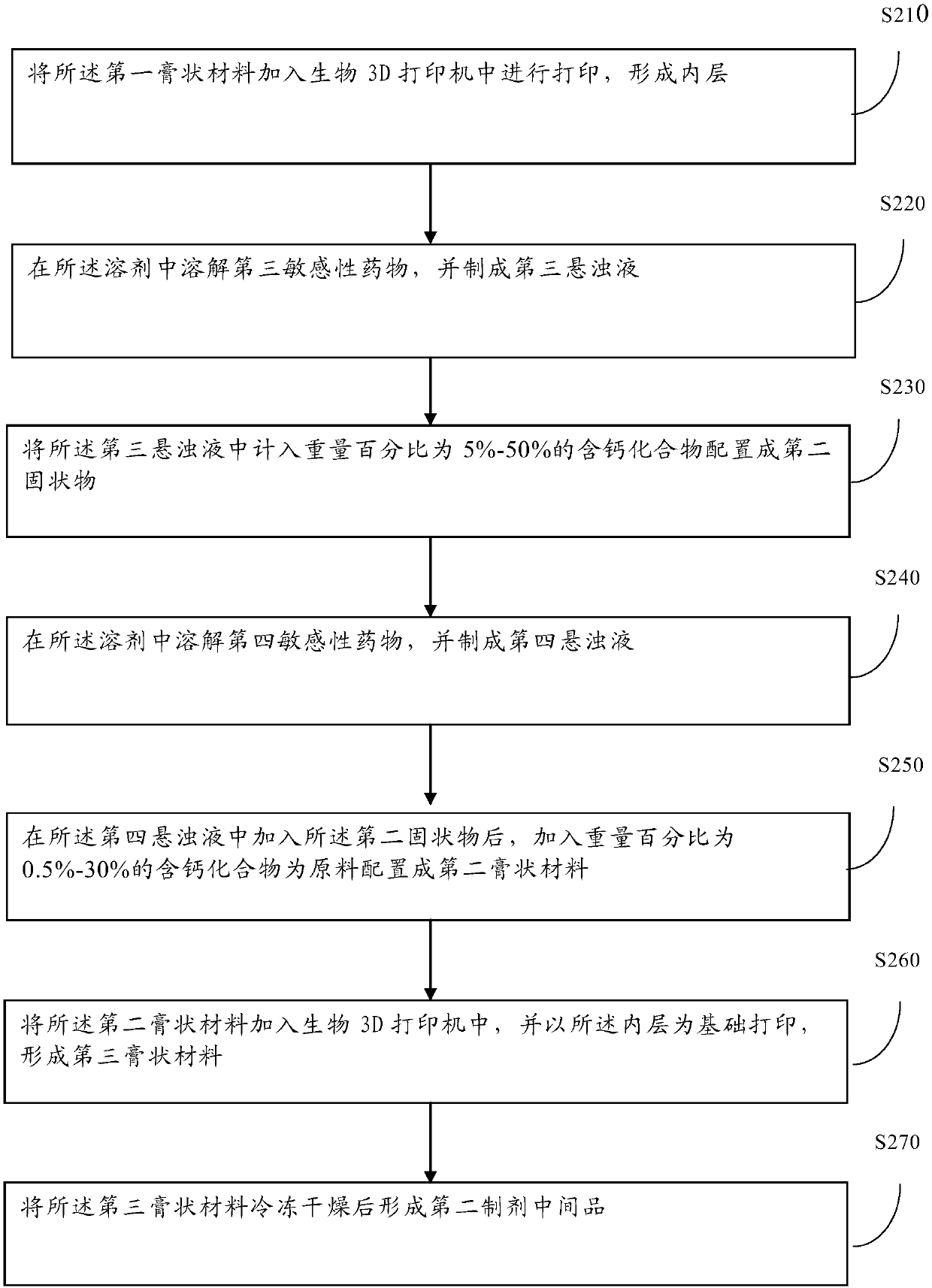
[0048] In order to realize the use of more moderately sensitive drugs and further improve the sustained-release effect, the embodiment of the present application also provides a method for manufacturing injection-type preparations by 3D printing artificial bones, such as figure 2 As shown, the method includes:
[0049] Step 210: adding the first paste material into a biological 3D printer for printing to form an inner layer;
[0050] Specifically, the first paste material that meets the input requirements of the biological 3D printer is added to the biological 3D printer for 3D printing. Since the 3D printing technology already belongs to the basic technology, the embodiment of the present application will not elaborate on the printing process and details of the 3D printer. method. It should be noted that the structure printed by the above 3D printer should match the bone defect required by the target object. That is to say, through the personalized characteristics of 3D pri...
Embodiment 3
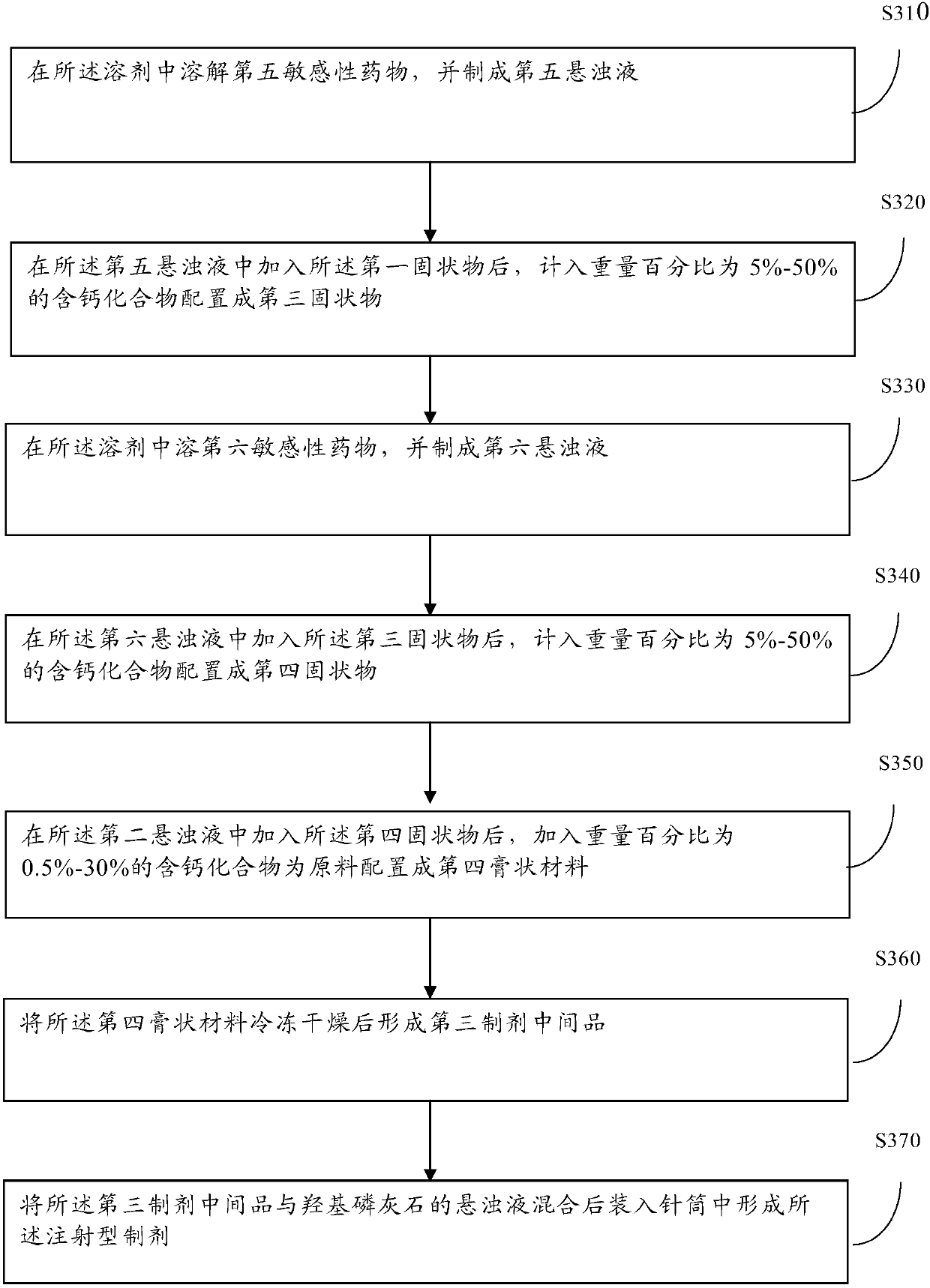
[0065] In order to realize the use of more sensitive drugs and further improve the sustained-release effect, the embodiment of this application also provides a method for manufacturing injection-type preparations by 3D printing artificial bone, please refer to image 3 , the method includes:
[0066] Step 310: dissolving the fifth sensitive drug in the solvent, and making a fifth suspension;
[0067] Step 320: After adding the first solid substance into the fifth suspension, add the calcium-containing compound with a weight percentage of 5%-50% to form a third solid substance.
[0068] Step 330: dissolving the sixth sensitive drug in the solvent, and making a sixth suspension;
[0069] Step 340: After adding the third solid substance into the sixth suspension, add the calcium-containing compound with a weight percentage of 5%-50% to form a fourth solid substance.
[0070] Step 350: After adding the fourth solid matter into the second suspension, add a calcium-containing comp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com