Liposome capable of improving drug release and preparation method of liposome
A technology of liposomes and blank liposomes, which is applied in liposome delivery, drug combination, and pharmaceutical formulations, etc., and can solve problems such as reduced therapeutic effect, low solubility, and slow drug release speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
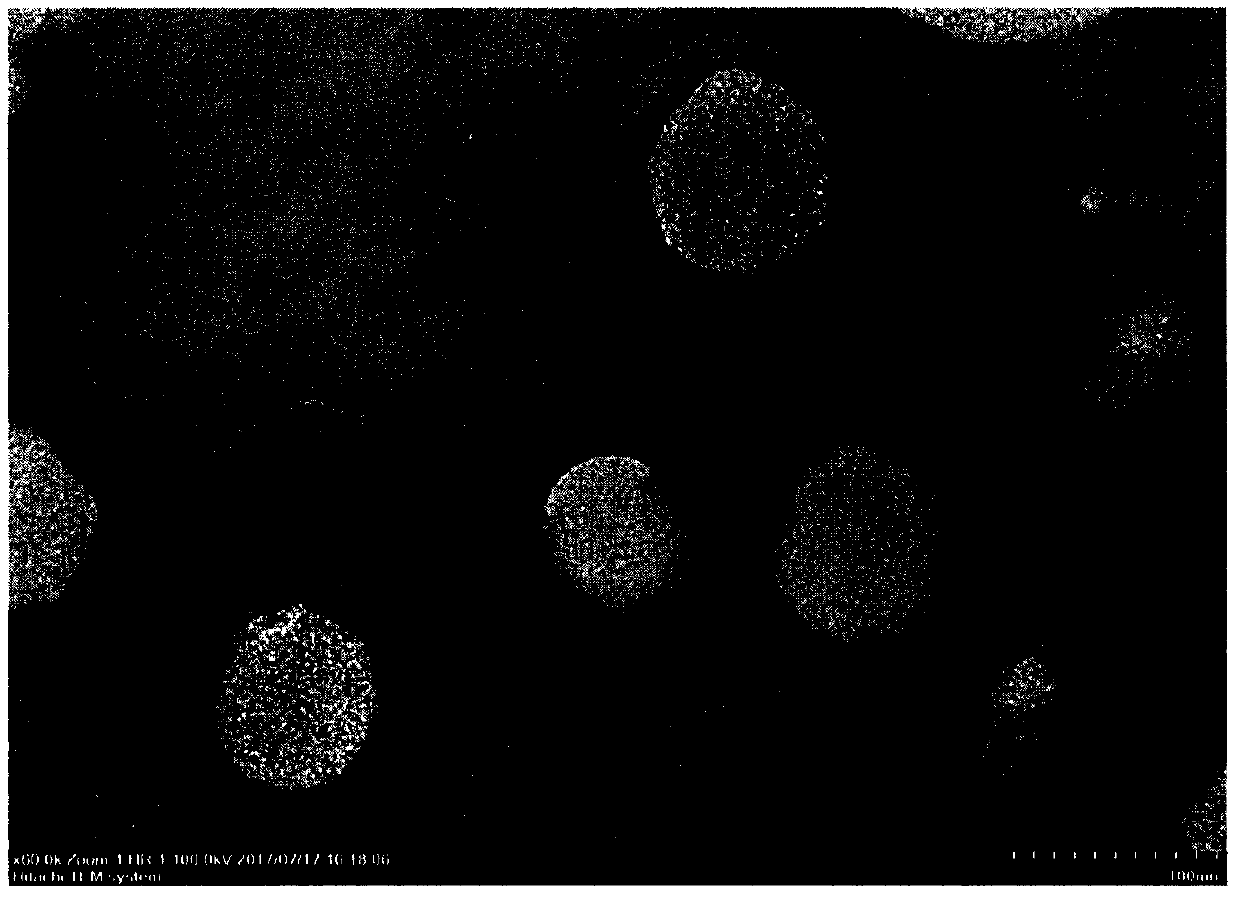
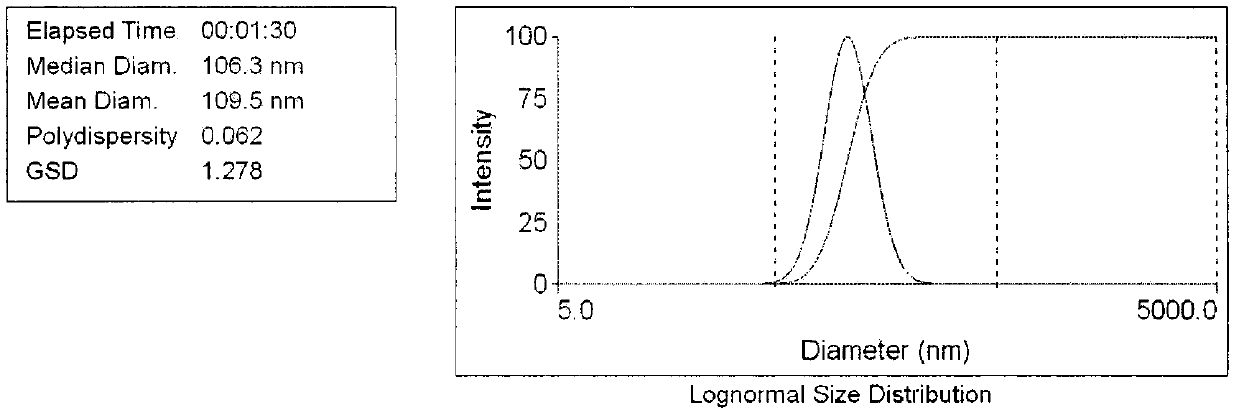
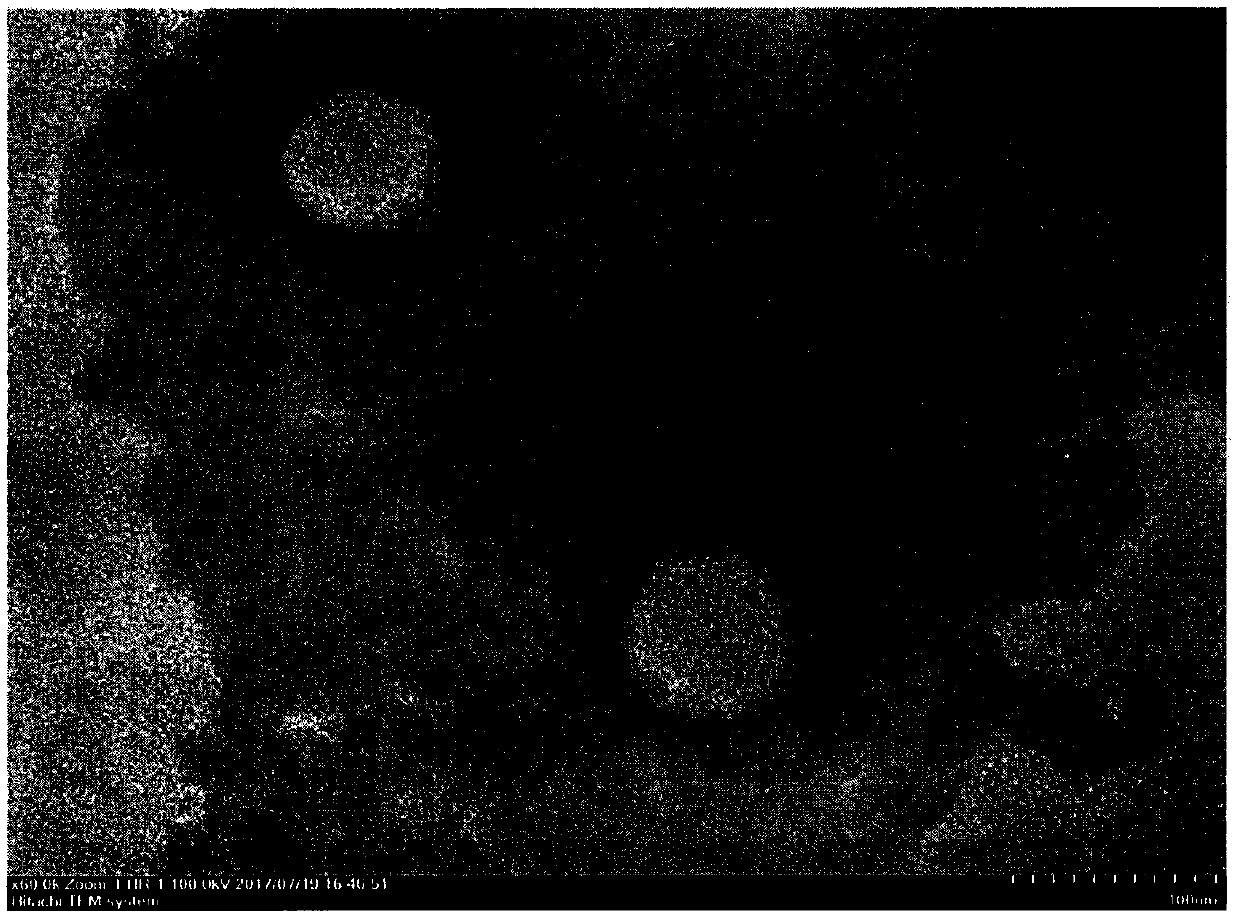
Image
Examples
Embodiment 1
[0030] Embodiment 1: Preparation of drug-loaded liposomes by ammonium sulfate gradient method
[0031] a. Preparation of blank liposomes
[0032] Prepare blank liposomes by ethanol injection method, weigh hydrogenated soybean phospholipids (HSPC) 420mg, cholesterol (Chol) 140mg, polyethylene glycol 2000-distearoylphosphatidylethanolamine (PEG) 2000 -DSPE) 10 mg was dissolved in an appropriate amount of absolute ethanol to obtain a lipid ethanol solution, which was injected into a 0.3 mol / L ammonium sulfate solution at 60°C, stirred at high speed for 5 minutes, and the ethanol was removed by rotary evaporation in a water bath at 60°C. Pass through polycarbonate membranes of 0.8, 0.4, 0.2, and 0.1 μm in sequence at 60°C to reduce particle size and particle size distribution, use pH 6.5 phosphate buffer as the dialysis medium, and dialyze 10 times the volume through an ultrafiltration device To remove the ammonium sulfate in the external water phase, the blank liposomes were obt...
Embodiment 2
[0035] Embodiment 2: Preparation of drug-loaded liposomes by carboxymethylcyclodextrin triethylamine gradient method
[0036] a. Preparation of carboxymethyl-β-cyclodextrin triethylamine salt
[0037] Prepare carboxymethylcyclodextrin triethylamine salt by ion exchange method, weigh 9g carboxymethyl-β-cyclodextrin sodium and dissolve it in an appropriate amount of purified water, load the sample through a cation exchange resin column, elute with purified water, and remove the sodium The salt is replaced by the free carboxylic acid. Measure the conductivity of the eluate or detect the pH of the eluate during the elution process, and collect the components with pH<5. Titrate the collected eluent with triethylamine, monitor the pH of the solution with a pH meter, and titrate to a pH of 5.5-6.0, accurately record the volume of triethylamine, and dilute with purified water. Carboxymethylcyclodextrin triethylamine is produced at a concentration of about 0.1 mol / l.
[0038] b. Prepa...
Embodiment 3
[0042] Example 3: Encapsulation efficiency of drug-loaded liposomes prepared by different gradient methods
[0043] According to the method described in embodiment 1, 2, prepare the liposomes of encapsulating different medicines respectively, and measure the irinotecan hydrochloride liposomes, vincristine sulfate liposomes and doxorubic acid hydrochloride prepared by two kinds of gradient methods respectively See Table 1 below for the encapsulation efficiency of Bixing liposomes.
[0044] Table 1 entraps the drug-loaded liposomes prepared by different preparation methods to entrap different drugs
[0045]
[0046] The results showed that the above three drug-loaded liposomes prepared by carboxymethylcyclodextrin triethylamine gradient method could all achieve higher encapsulation efficiency.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com