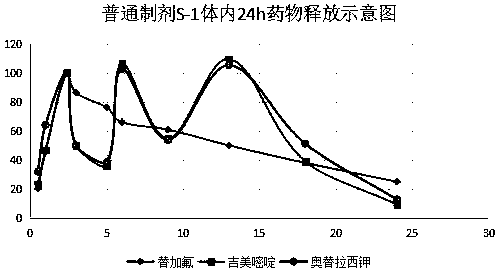
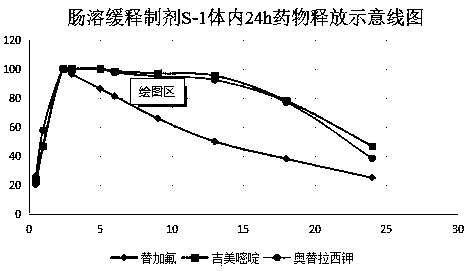
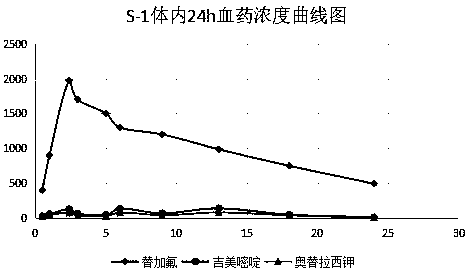
Enteric extended-release tegafur-gimeracil-potassium oxonate preparation, and preparation method thereof
A technology for sustained-release preparations and sustained-release tablets, which is used in the field of tablets, capsules, and solid oral enteric-coated extended-release preparations to achieve stable blood drug concentrations and reduce adverse reactions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Example 1: S-1 enteric-coated sustained-release tablets (skeleton type)
[0073] Material No.
1
2
3
20mg
40mg
80mg
Gimeracil
5.8mg
11.6mg
23.2mg
19.6mg
39.2mg
78.4mg
HPMC
20mg
80mg
150mg
MCC
20mg
80mg
200mg
EC
15mg
50mg
100mg
25mg
60mg
120mg
10mg
30mg
60mg
5mg
10mg
20mg
Acrylic resin No. Ⅱ
25mg
100mg
200mg
[0074] The preparation steps are:
[0075] ①Preparation of sustained-release tablet cores: Gymeracil, oteracil potassium and a certain amount of pharmaceutical excipients were crushed separately and passed through an 80-mesh standard sieve, weighed according to the prescription amount, fully mixed together, and then added a certain amount of 65% Ethanol solution is used as a binder to pre...
Embodiment 2
[0079] Example 2: S-1 enteric-coated sustained-release capsules (skeleton type)
[0080] Material No.
1
2
3
Tegafur
20mg
40mg
80mg
Gimeracil
5.8mg
11.6mg
23.2mg
19.6mg
39.2mg
78.4mg
HPMC
20mg
80mg
150mg
MCC
20mg
80mg
200mg
EC
15mg
50mg
100mg
25mg
60mg
120mg
10mg
30mg
60mg
5mg
10mg
20mg
[0081] ①Preparation of sustained-release capsule core: Gymeracil, oteracil potassium and a certain amount of pharmaceutical excipients were crushed separately and passed through an 80-mesh standard sieve, weighed according to the prescription amount, fully mixed together, and then added a certain amount of 65% Ethanol solution is used as a binder to prepare soft materials, passed through a 20-mesh standard sieve, dried at 60°C for 3 hours, and...
Embodiment 3
[0084] Example 3: S-1 enteric-coated sustained-release tablets (film-coated type)
[0085] Material No.
1
2
3
Tegafur
20mg
40mg
80mg
Gimeracil
5.8mg
11.6mg
23.2mg
Oteracil Potassium
19.6mg
39.2mg
78.4mg
HPMC
20mg
80mg
150mg
MCC
20mg
80mg
200mg
EC
15mg
50mg
100mg
MCC
25mg
60mg
120mg
pvp
10mg
30mg
60mg
5mg
10mg
20mg
Acrylic resin No. Ⅱ
25mg
100mg
200mg
Acrylic resin No.Ⅲ
25mg
100mg
200mg
[0086] The preparation steps are:
[0087] ①Preparation of sustained-release tablet cores: Gymeracil, oteracil potassium and a certain amount of pharmaceutical excipients were crushed separately and passed through an 80-mesh standard sieve, weighed according to the prescription amount, fully mixed together, and then added a certain amount of 65% E...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com