AIE luminogens for visualization and treatment of cancer
A luminophore and fluorescence technology, applied in luminescent materials, chemical instruments and methods, preparations for in vivo experiments, etc., can solve the problems of non-selective staining of cancer cells and fluorescence quenching
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0168] Characterization: Steady-state fluorescence spectra were recorded on a Perkin Elmer LS 55 spectrometer. Fluorescence images were collected on an Olympus BX 41 fluorescence microscope.
[0169] Cell culture: at 37 °C in 5% CO 2 HeLa cells were cultured in MEM containing 10% FBS and 1% antibiotics (100 units / mL penicillin and 100 g / mL streptomycin) in a humidity incubator. 5% CO at 37°C 2 COS-7, MDA-MB-231, MCF-7 and MDCK-II cells were cultured in DMEM containing 10% FBS and antibiotics (100 units / mL penicillin and 100 g / mL streptomycin) in a humidity incubator.
[0170] Cell Imaging: Two different types of cells were grown overnight on 35mm Petri dishes fitted with coverslips. Live cells were incubated with 200 nM TPE-IQ-2O for 1, 5, 10 and 20 minutes. In a typical experiment, 2 μL of a 10 mM TPE-IQ-2O stock solution in DMSO is first diluted to 2 mL with cell culture medium and then further diluted to the desired concentration. Cells were then imaged under a fluores...
Embodiment 2
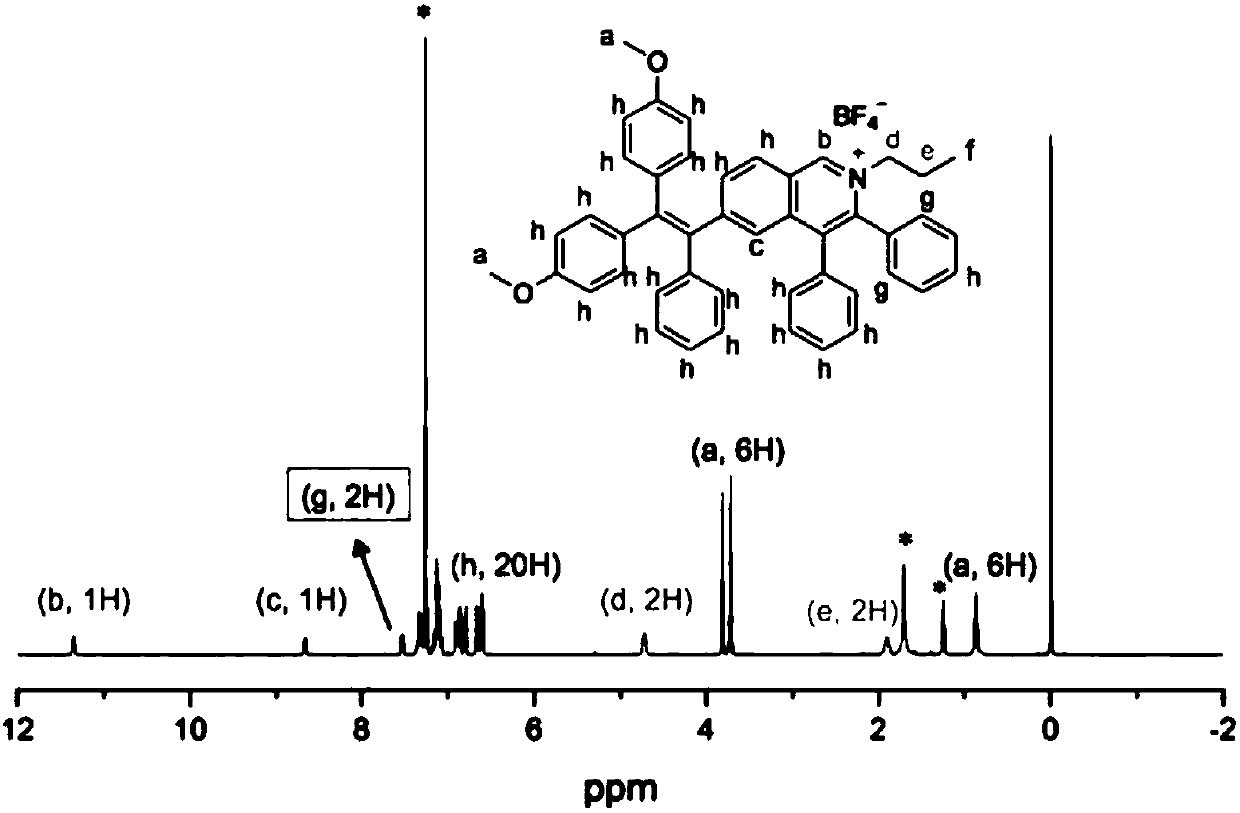
[0189] Preparation of Structure II (DPA-IQ): Briefly, [RhCp*Cl 2 ] 2 (2.0mol%), AgBF 4 (0.30mmol), Cu(OAc) 2 (0.30mmol), 4-(diphenylamino)benzaldehyde (0.36mmol), diphenylacetylene (0.30mmol), propylamine (0.45mmol) reaction mixture in 2.5ml tert-amyl alcohol was heated at 110°C Stir for 3 hours. After removal of solvent, the residue was purified by alumina column chromatography using dichloromethane / methanol (100:1 v / v) as eluent to give pure product as yellow solid in 60% yield.
[0190] 1 H-NMR (400MHz; d 6 -DMSO)δ H 9.77(s,1H),8.29(d,2H,J=9.6Hz),7.44-7.36(m,10H),7.30-7.24(m,6H),7.11-7.10(m,3H),7.04-7.02( m,2H),6.43(s,1H),4.18(t,2H,J=7.2Hz),1.75-1.69(m,2H),0.73(t,2H,J=7.2Hz)ppm. 13 C-NMR (400MHz; CDCl 3 )δ C 155.0,149.1,144.4,143.3,139.7,135.4,133.7,133.6,131.5,130.4,130.2,130.0,128.8,128.2,128.1,127.0,126.8,123.5,122.1,109.5,59.3,02 11 B-NMR-1.327ppm. 19 F-NMR-148.3ppm.MALDI-MS DPA-IQ cation (C 36 h 31 N 2 + ): The calculated value is 491.2482, and the me...
Embodiment 3
[0197] Preparation of Structure III (TPA-IQ): The synthesis method is similar to DPA-IQ.
[0198] 1 H-NMR (400MHz; d 6 -DMSO)δ H 10.29(s,1H),8.66(d,1H,J=8.8Hz),8.43(d,1H,J=8.4Hz),7.60-7.58(m,3H),7.49(s,2H),7.40-7.28 (m,12H),7.15-7.08(m,6H),7.00(d,2H,J=8.4Hz),4.36(t,2H,J=7.2Hz),1.85-1.80(m,2H),0.79( t,3H,J=7.2Hz)ppm. 13 C-NMR (400MHz; d 6 -DMSO)δ C 149.3,149.1,147.7,146.3,144.3,137.9,137.7,133.5,131.4,131.1,130.4,130.3,130.1,130.0,129.8,128.7,128.5,128.3,125.5,125.2,124.3,121.6,120.8,60.0,23.7, 10.5ppm. 11 B-NMR (128MHz; d 6 -DMSO)-1.32ppm. 19 F-NMR (376MHz; d 6 -DMSO)-148.3ppm. The cation of MALDI-MS TPA-IQ (C 42 h 35 N 2 + ): The calculated value is 567.2795, and the measured value is 567.2771.
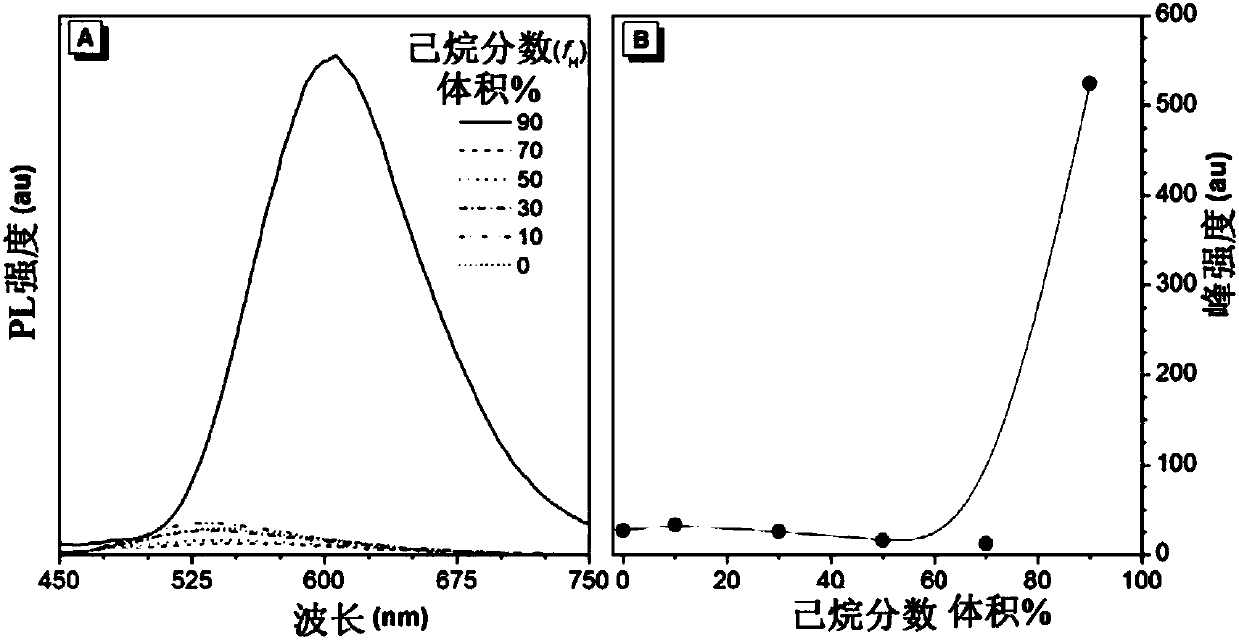
[0199] TPA-IQ exhibits typical AIE features, as shown in Figure 10. The DMSO solution of TPA-IQ hardly emits light, while the aggregated state powder of the substance emits strong yellow-orange fluorescence when excited by UV. Figure 10 shows the UV absorption spec...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com