Iohexol intermediate impurity preparation method and application
An intermediate, iohexol technology, applied in the field of drug synthesis, to achieve the effect of short steps, simple preparation process and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
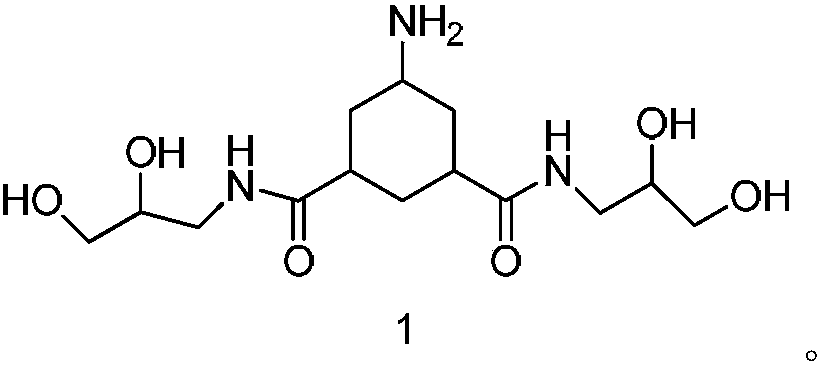
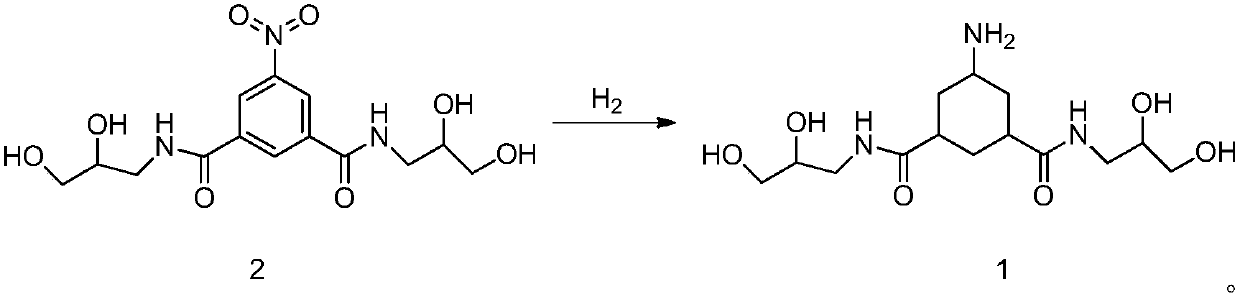
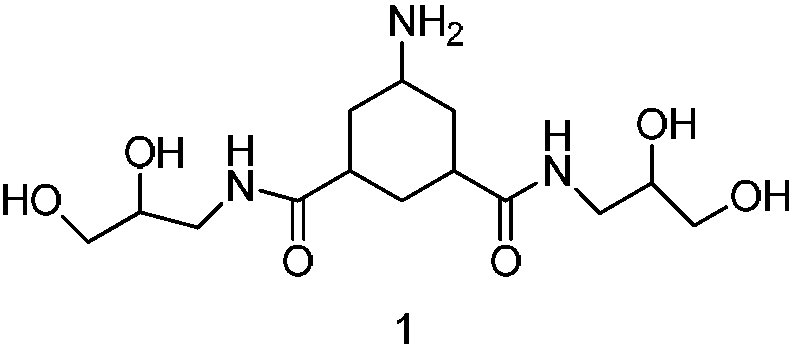
[0057] The preparation of embodiment 1, formula 1 compound
[0058] The formula 2 compound (10g, 0.028mol) was added to the autoclave, followed by methanol (40g), acetic acid (25g) and Rh / C (1g), 5.5MPa H 2 Reaction at room temperature, TLC monitoring completion of the reaction, suction filtration, the filtrate was concentrated to dryness under reduced pressure at 40°C to obtain 8.6 g of the crude compound of formula 1, the crude product weight yield was 86%, and the purity was 87%.
Embodiment 2
[0059] Embodiment 2, the purification of formula 1 compound
[0060]Dissolve the compound of formula 1 (0.5g, 0.0014mol) in methanol (1ml), add silica gel (0.5g, 200-300 mesh) to mix the sample, 10g of silica gel (200-300 mesh) for column, first use 25mL ethyl acetate Elution, followed by elution with 85mL of ethyl acetate:methanol with a volume ratio of 1:1, and finally with 50mL of ethyl acetate:methanol:ammonia with a volume ratio of 1:2:0.01 for elution, TLC detection and collection , the product was concentrated to dryness under reduced pressure at 40°C to obtain 0.4 g of off-white solid, which was the pure compound of formula 1. The total molar yield is 85.8%, and the purity is 98%.
Embodiment 3
[0061] Embodiment 3, confirmation of the structure of the compound of formula 1 in embodiment 2
[0062] Mass Spectrum: ESI-MS(m / z): 334.1[M+H] +
[0063] High resolution mass spectrum: m / z: 334.1974[M+H] + ;Molecular formula: C14H27N3O6
[0064] Proton NMR spectrum: 1 HNMR(400MHz,DMSO-d6)δ7.72-7.70(t,2H),5.10-4.05(bs,4H),3.46-3.43(m,2H),3.27-3.22(m,4H),3.16-3.12( m,2H),2.98-2.93(m,2H),2.55-2.53(m,1H),2.23-2.19(t,2H),1.76-1.74(d,2H),1.63-1.60(m,1H), 1.37-1.41(m,1H),1.09-1.03(m,2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com