Method for synthesizing 2,4-diamino-6-chloropyrimidine
A synthetic method, diamino technology, applied in the direction of organic chemistry, can solve the problems of low yield, high cost, complicated operation, etc., and achieve the effect of improving product purity and yield, increasing yield, and high safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
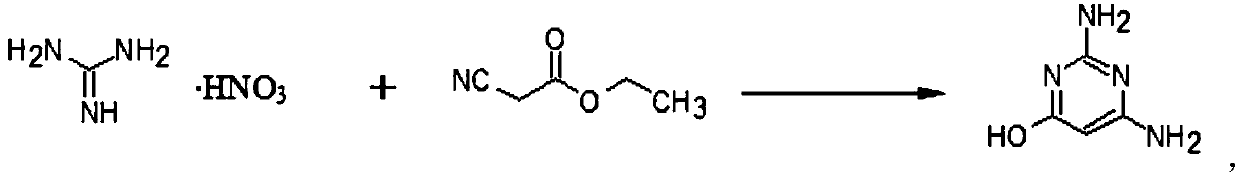
[0032] Embodiment 1: the preparation of DAHP
[0033] Add 300ml of methanol, 110g of guanidine nitrate, and 55g of sodium methoxide into a four-neck flask, heat, stir for 1 hour, and add methyl cyanoacetate dropwise under reflux. After reflux for 4 hours, distill off and recover methanol (applicable), add Water 800ml, adjust pH=9 with hydrochloric acid, then adjust pH=7 with 50% acetic acid, cool to 5-10°C, filter, wash with water, dry to obtain 120g of DAHP dry product, yield 95%, content 99.10 %.
Embodiment 2
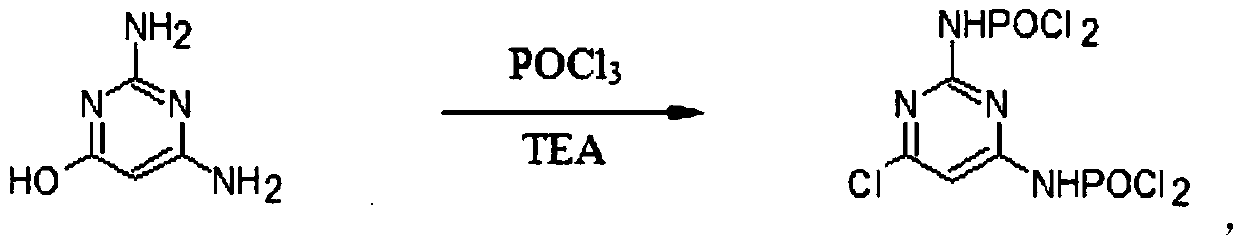
[0036] Embodiment 2: the preparation of DACP
[0037] Add 12.6g of DAHP and 76.5g of phosphorus oxychloride into a 250ml three-necked flask, and add 20g of triethylamine TEA dropwise while stirring, (note: the temperature during the dropping process should not exceed 35°C), and the temperature will rise to 95-110°C after the addition. ℃, the reaction was incubated for 2 hours until DAHP disappeared.
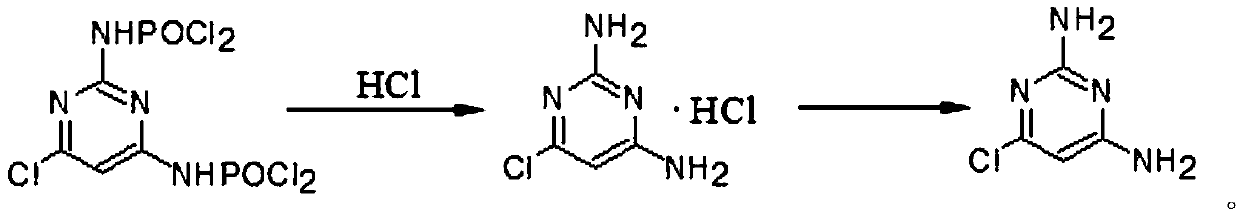
[0038] Add 100ml of water into a 500ml four-neck flask, cool down to 5°C, slowly add the chlorinated mixture while stirring (note: the temperature should be controlled within 50°C), and then keep it warm at 55-60°C for 2 hours, about 1 hour Afterwards, a large amount of material was precipitated, and a sample was taken to determine the reaction end point. After reaching the end point, cool down to 0-5°C, keep warm for 2 hours, filter, and drain as much as possible, then add the filter cake to 50ml of water, heat up to 70°C, dissolve, add 0.5g of activated carbon, filter, and use...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com