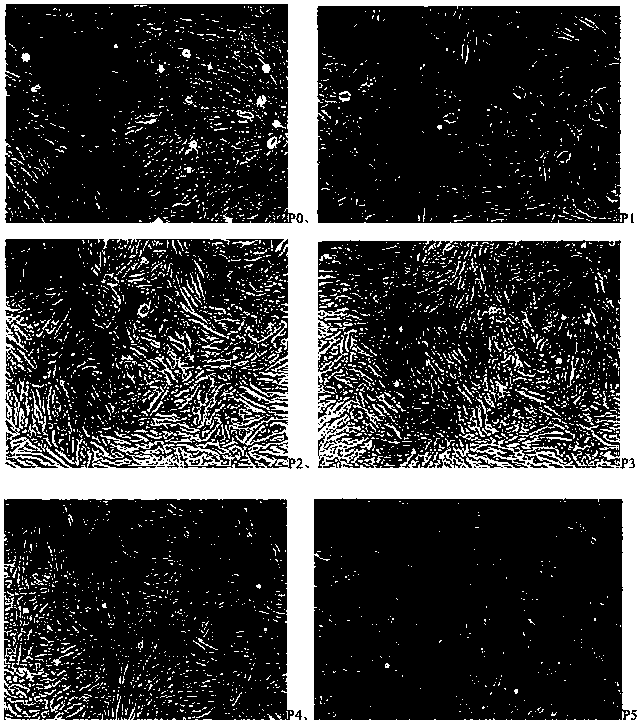
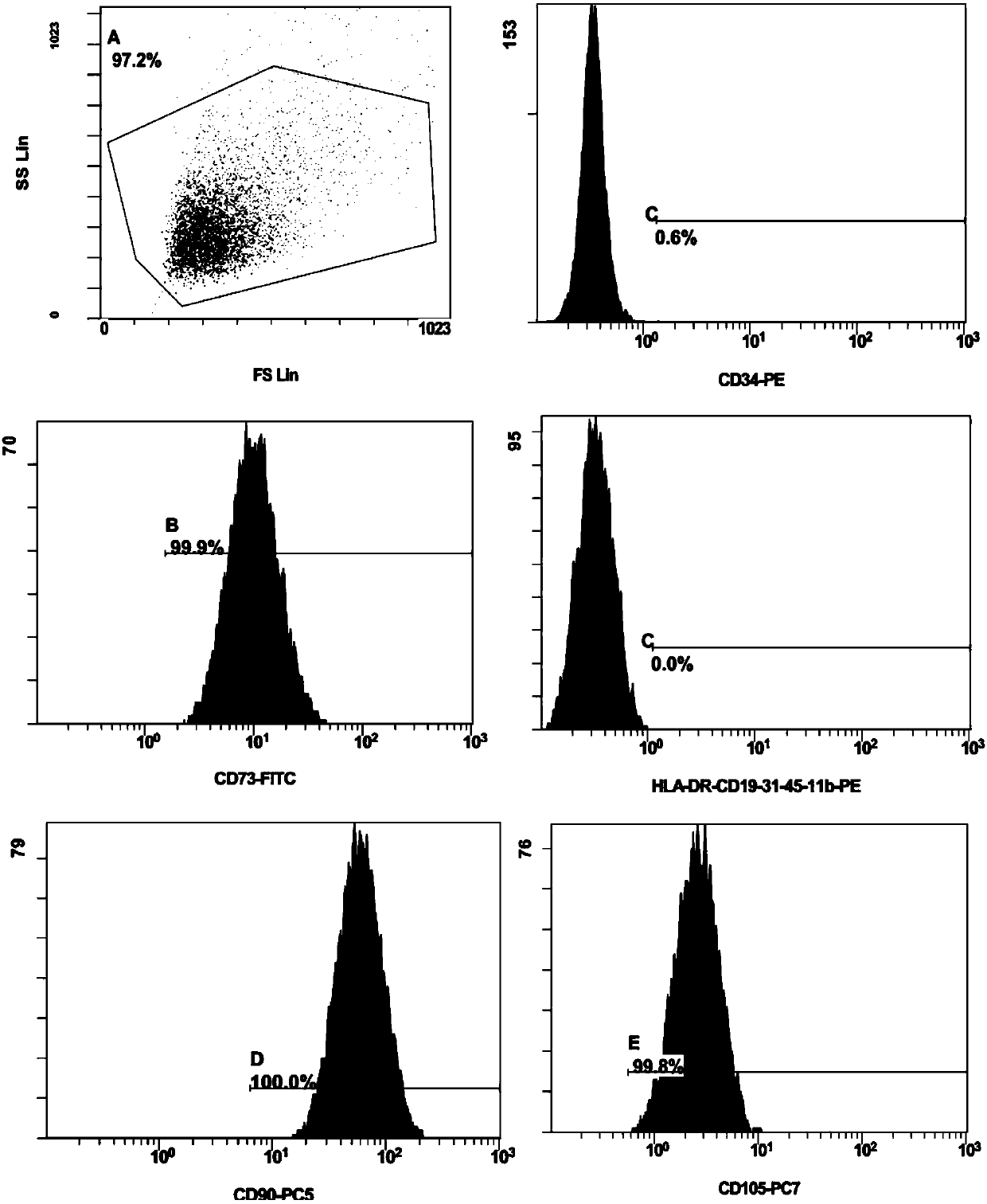
Method for separating adipose-derived stem cells from fat and preparation in use
A technology of mesenchymal stem cells and fat, applied in the field of isolation and culture of autologous adipose-derived mesenchymal stem cells, can solve the problems of inapplicable acquisition of a large number of adipose-derived mesenchymal stem cells, low cell yield, and low cell viability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0111] Example 1: Isolation and culture of adipose-derived mesenchymal stem cells
[0112] (1) Put the fat sample mixed with swelling fluid obtained by liposuction into a centrifuge tube, and centrifuge at 800rpm for 3min;
[0113](2) Remove the top layer of fat, and pipette the suspended fat particles and the bottom cell sediment respectively, and set aside;
[0114] (31) Add mixed enzyme (0.05% collagenase type I+0.05% collagenase type II+0.03% neutral protease+0.03% DNase) with volume ratio 1: 1 to the fat granule gained in step (2), at 37 Centrifuge at 100rpm for 30min; after digestion, add an equal volume of complete medium to stop digestion, centrifuge at 1500rpm for 5min, discard the supernatant, and resuspend the cell pellet in PBS;
[0115] (32) Resuspend the bottom cell pellet obtained in step (2) with PBS, add an equal amount of erythrocyte lysate and let it stand for 10 minutes to lyse; after the erythrocytes are fully lysed, centrifuge at 1500 rpm for 5 minutes...
Embodiment 2
[0123] Example 2: Isolation and culture of adipose-derived mesenchymal stem cells
[0124] (1) Put the fat sample mixed with swelling fluid obtained by liposuction into a centrifuge tube, and centrifuge at 500rpm for 5min;
[0125] (2) Remove the top layer of fat, and pipette the suspended fat particles and the bottom cell sediment respectively, and set aside;
[0126] (31) Add mixed enzyme (0.05% collagenase type I+0.05% collagenase type II+0.03% neutral protease+0.03% DNase) with volume ratio 1:1.2 in the fat granule gained in step (2), at 37 Centrifuge at 100rpm for 20min; after digestion, add an equal volume of complete medium to stop digestion, centrifuge at 2000rpm for 3min, discard the supernatant, and resuspend the cell pellet in PBS;
[0127] (32) Resuspend the bottom cell pellet obtained in step (2) with PBS, add an equal amount of erythrocyte lysate and let it stand for 5 minutes to lyse; after the erythrocytes are fully lysed, centrifuge at 2000 rpm for 3 minute...
Embodiment 3
[0134] Example 3: Isolation and culture of adipose-derived mesenchymal stem cells
[0135] (1) Put the fat sample mixed with swelling fluid obtained by liposuction into a centrifuge tube, and centrifuge at 1000rpm for 2min;
[0136] (2) Remove the top layer of fat, and pipette the suspended fat particles and the bottom cell sediment respectively, and set aside;
[0137] (31) Add mixed enzymes (0.05% collagenase type I+0.05% collagenase type II+0.03% neutral protease+0.03% DNase) with a volume ratio of 1:0.8 to the fat granules obtained in step (2), at 37 Centrifuge at 100rpm for 40 minutes; after digestion, add an equal volume of complete medium to stop digestion, centrifuge at 1000 for 8 minutes, discard the supernatant, and resuspend the cell pellet in PBS;
[0138] (32) Resuspend the bottom cell pellet obtained in step (2) with PBS, add an equal amount of erythrocyte lysate and let it stand for 15 minutes to lyse; after the erythrocytes are fully lysed, centrifuge at 100...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com