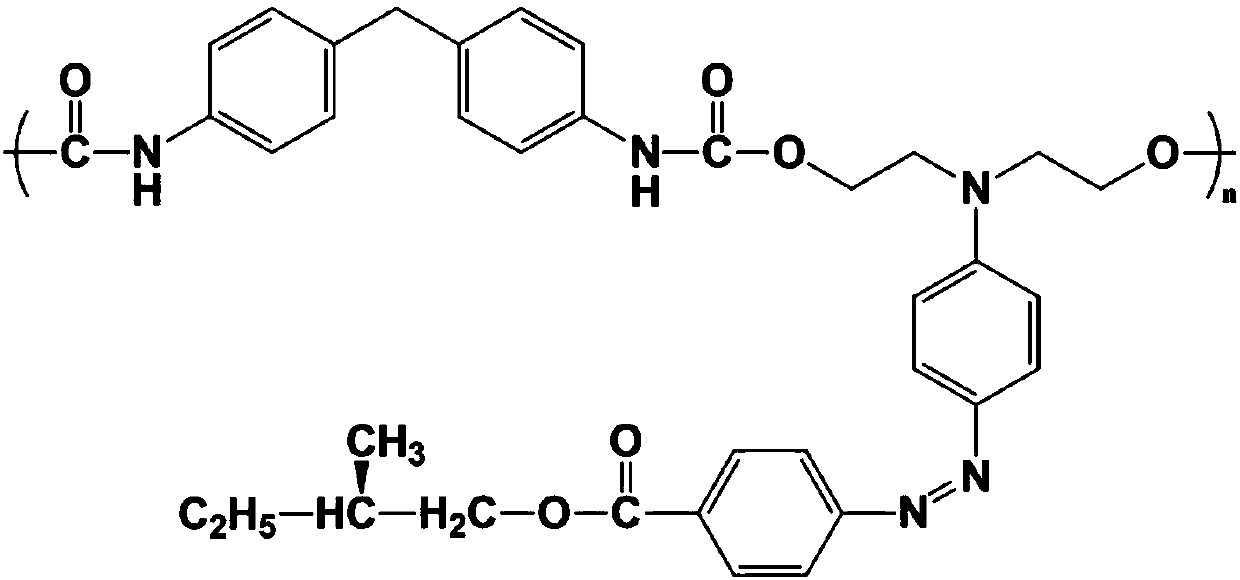
Chiral azobenzene polymer and production method thereof
A kind of technology of azo polymer and manufacturing method, applied in the direction of optical recording carrier, recording carrier material, etc., can solve the problems of complex storage process of holographic optical storage materials, inability to realize holographic optical storage of information, non-repeatable erasing and writing, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] (1) Preparation of S-2-methyl-1-bromobutane: Dissolve 1.02g of S-2-methyl-1-butanol in 40mL of dichloromethane and cool to 0°C, add 5.40g of tribromide Phosphorus was added dropwise to the above solution at a rate of 30 drops per minute. After the addition, the reaction was continued for 2 hours to obtain S-2-methyl-1-bromobutane;
[0019] (2) Preparation of 4-aminobenzoic acid S-2-methylbutyl ester: Add 3.02g 4-aminobenzoic acid and 2.91g S-2-methyl-1-bromobutane to 50mL dimethylformamide Add 3.04g potassium bicarbonate as a catalyst, and react at 50°C for 24h to obtain 4-aminobenzoic acid S-2-methylbutyl ester;
[0020] (3) Preparation of polyurethane: 2.51g diphenylmethane-4,4'-diisocyanate and 1.81g N,N-dihydroxyethylaniline were added to 25mL dimethylformamide, and then 50μL dilauric acid Butyltin is used as a catalyst and reacted at 80℃ for 12h to obtain polyurethane;
[0021] (4) Preparation of chiral azo polymer: add 1.21g of 4-aminobenzoic acid S-2-methylbutyl ester...
Embodiment 2
[0024] (1) Preparation of S-2-methyl-1-bromobutane: the same as the process in Example 1;
[0025] (2) Preparation of S-2-methylbutyl 4-aminobenzoate: the same as the process in Example 1;
[0026] (3) Preparation of polyurethane: 2.51g diphenylmethane-4,4'-diisocyanate and 1.81g N,N-dihydroxyethylaniline were added to 25mL dimethylformamide, and then 50μL dilauric acid Butyltin is used as a catalyst and reacted at 80℃ for 8h to obtain polyurethane;
[0027] (4) Preparation of chiral azo polymer: the same as the process in Example 1;
[0028] The structural formula of the obtained chiral polymer is as attached figure 1 As shown, the molecular weight is 1.73x10 4 Under 532nm interference laser irradiation, a clear holographic pattern can be stored on the surface of the chiral azo polymer film, and the formed holographic pattern can be erased and rewritten.
Embodiment 3
[0030] (1) Preparation of S-2-methyl-1-bromobutane: the same as the process in Example 1;
[0031] (2) Preparation of S-2-methylbutyl 4-aminobenzoate: the same as the process in Example 1;
[0032] (3) Preparation of polyurethane: 2.51g diphenylmethane-4,4'-diisocyanate and 1.81g N,N-dihydroxyethylaniline were added to 25mL dimethylformamide, and then 50μL dilauric acid Butyltin is used as a catalyst and reacted at 80℃ for 16h to obtain polyurethane;
[0033] (4) Preparation of chiral azo polymer: the same as the process in Example 1;
[0034] The structural formula of the obtained chiral polymer is as attached figure 1 As shown, the molecular weight is 3.73x10 4 Under 532nm interference laser irradiation, a clear holographic pattern can be stored on the surface of the chiral azo polymer film, and the formed holographic pattern can be erased and rewritten.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com