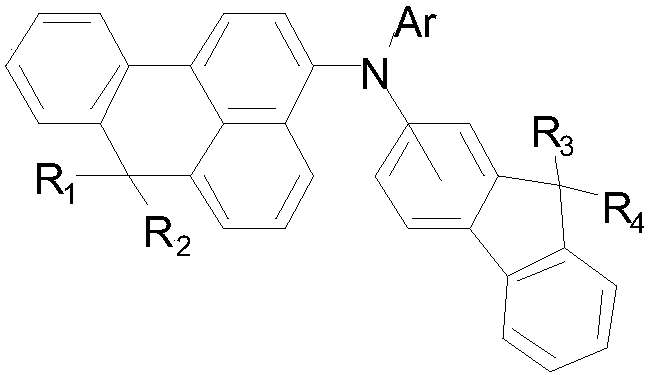
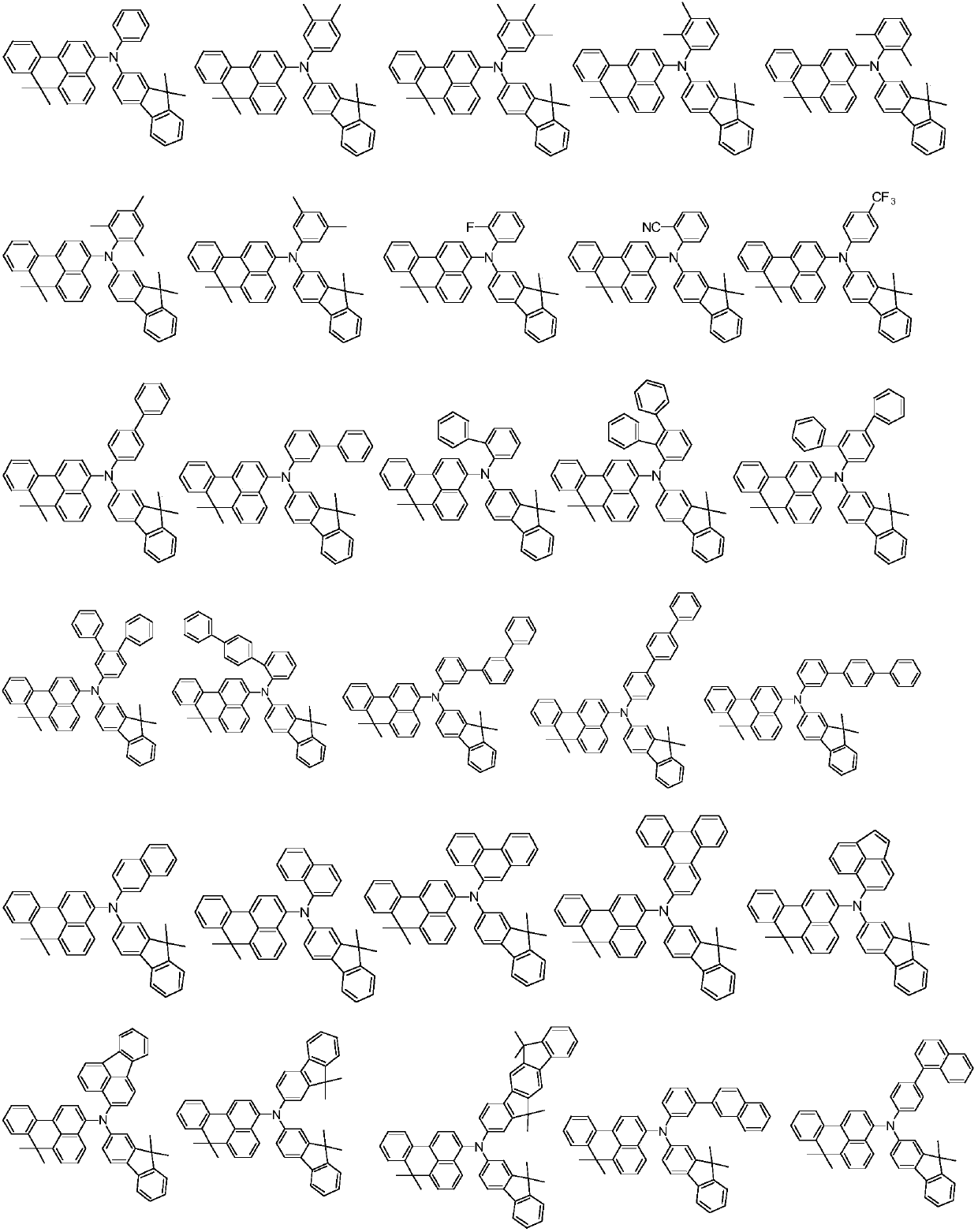
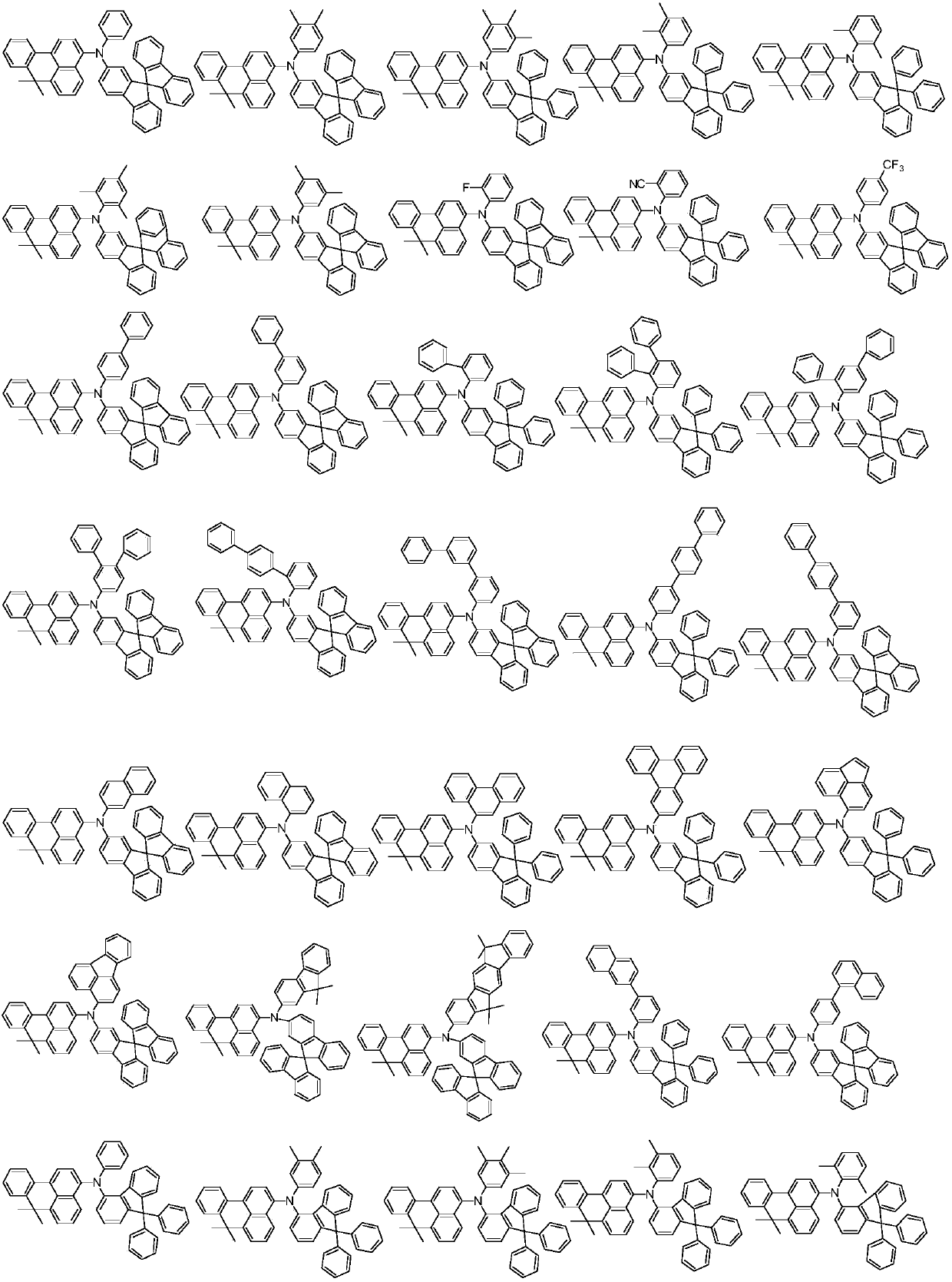
Benzoanthracene compound, preparation method of benzoanthracene compound, and organic electroluminescent device
A compound, benzanthracene technology, applied in the field of luminescent materials, can solve problems such as electronic imbalance, luminous efficiency and lifespan reduction, and achieve the effect of simple preparation method, high yield and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0041] The present invention also provides a preparation method of benzanthracene compound, comprising the following steps:
[0042] Synthesis of compound Ⅲ
[0043] Add compound I, compound II, Pd to the reaction vessel in sequence 2 (dba) 3 , P(t-Bu) 3 , NaOt-Bu, and toluene, react at 100°C, extract the organic matter with ether and water after the reaction, dry the organic layer with magnesium sulfate, and use silica gel column and recrystallization method to obtain compound III;
[0044] Synthesis of target compounds
[0045] Add compound III, compound IV, Pd to the reaction vessel in sequence 2 (dba) 3 , P(t-Bu) 3 , NaOt-Bu, and toluene, react at 100°C, extract the organic matter with dichloromethane and water after the reaction, and use silica gel column and recrystallization to obtain the target compound from the concentrated product after drying the organic layer with magnesium sulfate; The synthetic route is as follows:
[0046]
[0047] In the formula, R ...
Synthetic example 1
[0050] Synthesis of compound 1-1
[0051]
[0052] Add 2-bromo-9,9-dimethyl-9H-fluorene (37.7g, 138mmol), 7,7-dimethyl-7H-benzanthracen-3-ylamine (32.5g ,125.5mmol), Pd 2 (dba) 3 (5.74g, 6.3mmol), P(t-Bu) 3 (2.54g, 12.5mmol), NaOt-Bu (36.2g, 376.4mmol), and 1320mL of toluene were reacted at 100°C. After the reaction was completed, the organic matter was extracted with ether and water, and the organic layer was dried with magnesium sulfate, and the product was concentrated to obtain compound 1-1, 56.6 g (75%), by silica gel column and recrystallization. MS / FAB 451, calculated 451.23.
[0053] Synthesis of compound 1
[0054] Add bromobenzene (8.8g, 56.3mmol), compound 1-1 (21.2g, 46.9mmol), Pd 2 (dba) 3 (1.95g, 2.13mmol), P(t-Bu) 3 (0.86g, 4.3mmol), NaOt-Bu (12.3g, 128mmol), and 300mL of toluene were reacted at 100°C. After the reaction was completed, the organic matter was extracted with dichloromethane and water, and the concentrated product after the organic layer ...
Synthetic example 2
[0056] Synthesis of compound 2
[0057]
[0058] Using 4-bromo-biphenyl (13.2g, 56.6mmol), compound 1-1 (21.3g, 47.2mmol), Pd 2 (dba) 3 (1.97g, 2.15mmol), P(t-Bu) 3 (0.87g, 4.3mmol), NaOt-Bu (12.4g, 128.8mmol), toluene 450mL and other raw materials were synthesized according to compound 1 to obtain compound 2, 28.4g (75%). MS / FAB 603, calculated 603.29.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com