Method for preparing high-purity lithium sulfate from waste liquid recovered from hydrothermal method based lithium iron phosphate production
A technology of lithium iron phosphate and lithium sulfate, applied in the direction of lithium sulfate/sulfite, etc., can solve the problems affecting the quality of lithium products, pollution, lithium can not be fully recovered, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
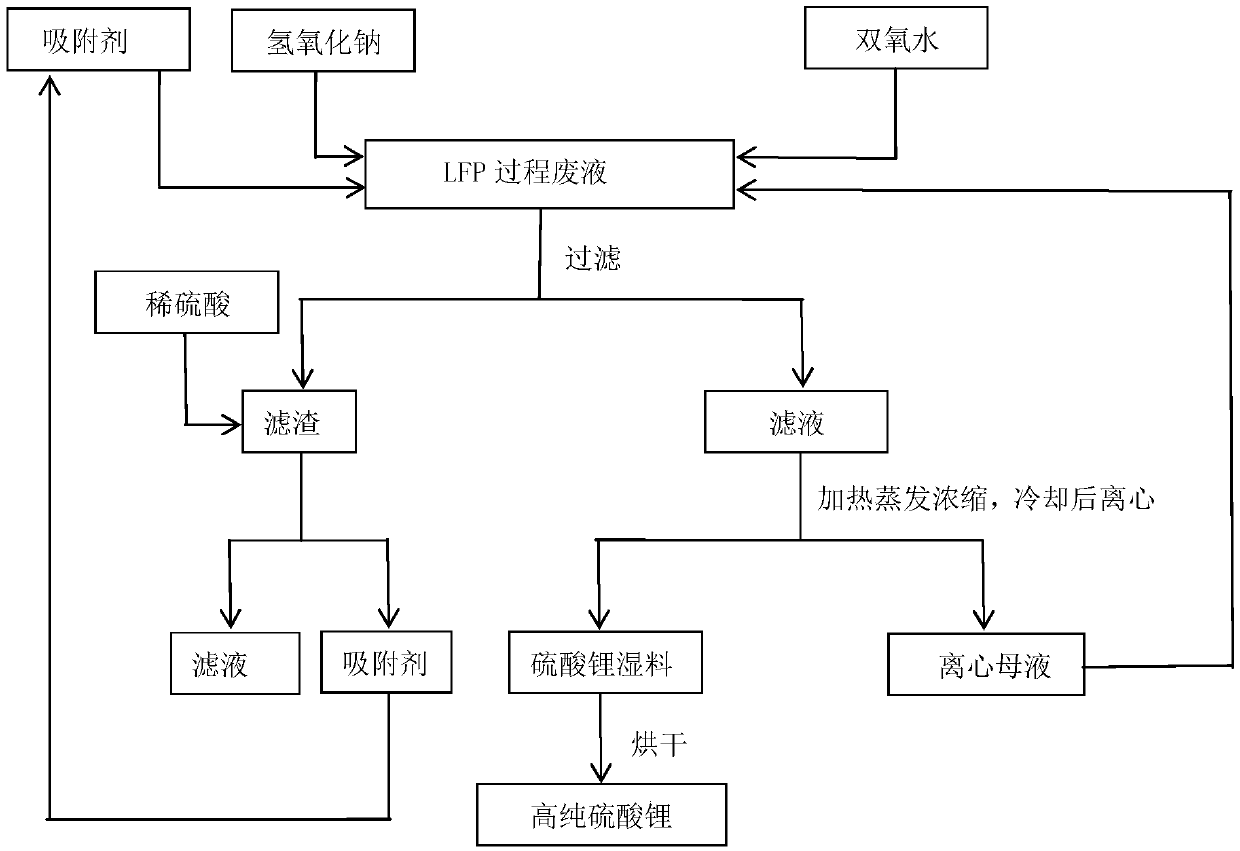
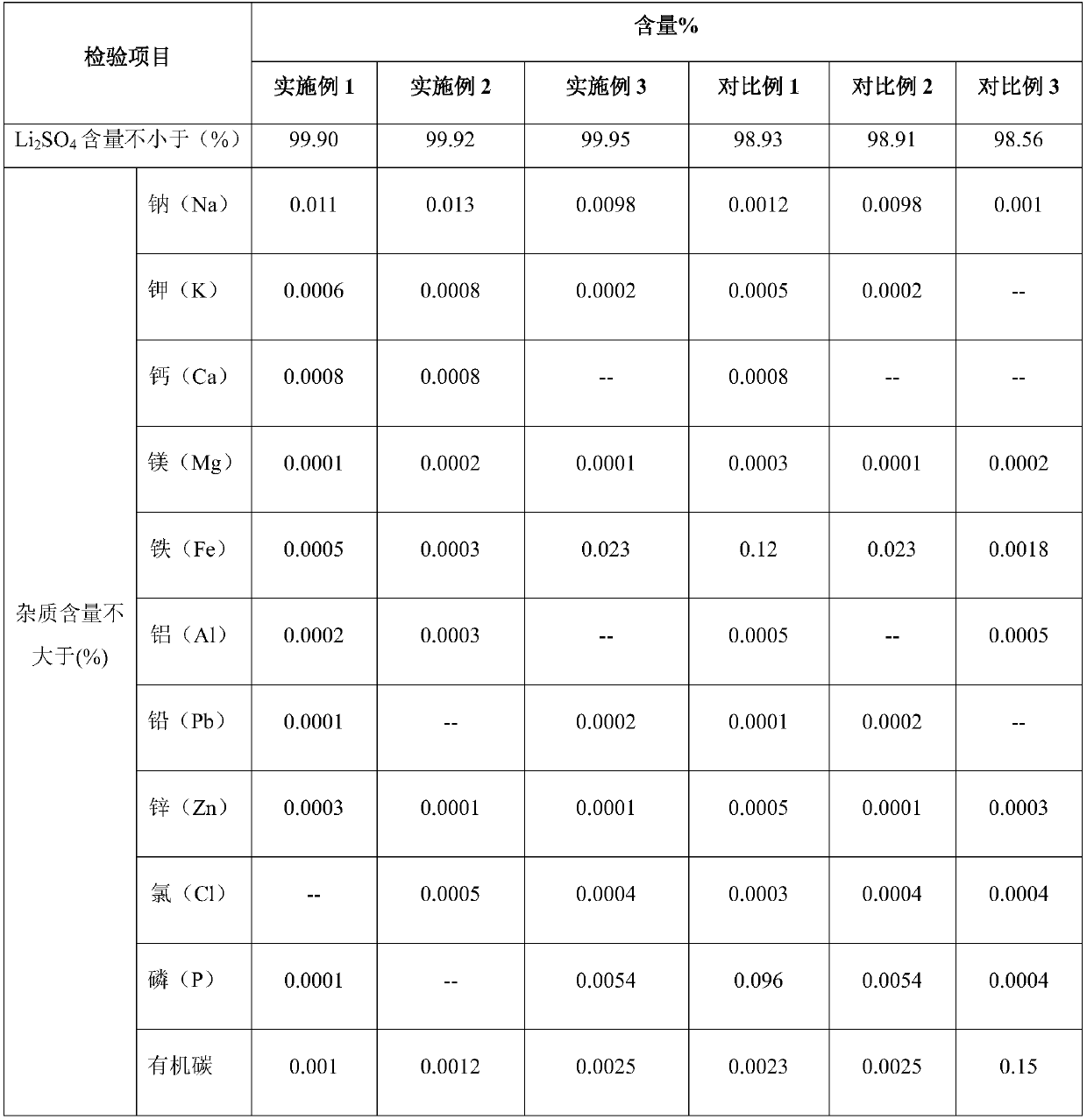
[0028] Get LFP process waste liquid 2L (pH 3.51, main component is Li + : 9.4g / L, Fe 2+ : 0.5g / L, ascorbic acid: 1g / L, furfural: 0.02g / L), add sodium hydroxide to it after boiling, adjust the pH value to 6.5, react for 15min; add 30% hydrogen peroxide 4.5ml, add activated carbon after reacting for 15min 20g, reacted for 15min and filtered to obtain 29.4g of filter residue. The filter residue is stirred and washed with 294ml of dilute sulfuric acid with a mass concentration of 10% for 15 minutes, and the solid obtained after filtration is used as an adsorbent for cyclic adsorption; the filtrate is heated and evaporated to concentrate a large amount of solids, and after cooling, it is centrifugally separated, and the mother liquor is returned to the raw material lithium iron phosphate From the waste liquid, the wet material was separated by centrifugation, put into an oven and baked at 110°C for 4 hours to obtain 83.1 g of high-purity anhydrous lithium sulfate. The direct reco...
Embodiment 2
[0030] Get LFP process waste liquid 3L (pH 3.45, main component is Li + : 8.9g / L, Fe 2+ : 0.6g / L, ascorbic acid: 0.1g / L, furfural: 0.82g / L), after boiling, add sodium hydroxide to it, adjust the pH value to 6, react for 18min; add 1.8ml of 30% hydrogen peroxide, react for 20min, then add Activated carbon 30g, reacted for 15min and filtered to obtain 41.4g of filter residue; the filter residue was stirred and washed with 414ml of 6% dilute sulfuric acid for 20min, and the solid obtained after filtration was used as an adsorbent for cyclic adsorption; the filtrate was heated, evaporated and concentrated until a large amount of solids were precipitated. After centrifugation, the mother liquor was returned to the raw material lithium iron phosphate waste liquid, and the wet material was centrifugally separated and put into an oven for drying at 120° C. for 5 hours to obtain 120.45 g of high-purity anhydrous lithium sulfate. The direct recovery rate of lithium in the waste liquid ...
Embodiment 3
[0032] Get LFP process waste liquid 10L (pH 3.49, main component is Li + : 8.9g / L, Fe 2+ : 0.56g / L, citric acid: 0.95g / L,), add 30% hydrogen peroxide 3.1ml, add sodium hydroxide to it after boiling, adjust the pH value to 7, react for 16min; add montmorillonite 120g after reacting for 15min After 20 minutes of reaction, 170 g of filter residue was obtained by filtration; the filter residue was stirred and washed with 1700 ml of 5% dilute sulfuric acid for 18 minutes, and the solid obtained after filtration was used as an adsorbent for cyclic adsorption; , the mother liquor was returned to the raw material lithium iron phosphate waste liquid, the wet material was centrifuged and put into an oven for 5.5 hours at 125°C to obtain 414.5g of high-purity anhydrous lithium sulfate. The direct recovery rate of lithium in the waste liquid of this embodiment is 59.3%, and the purity of the prepared high-purity anhydrous lithium sulfate is 99.95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com