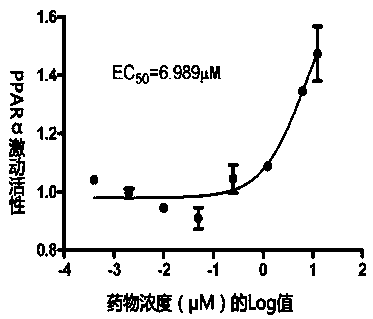
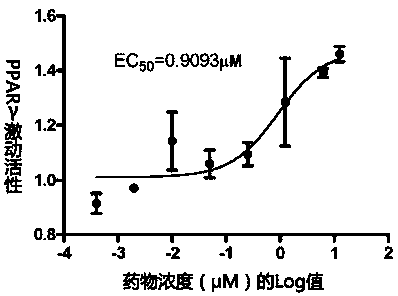
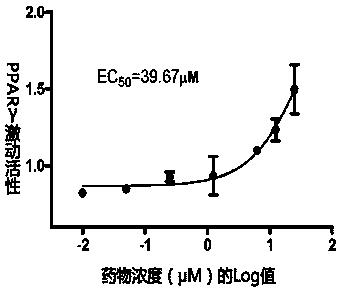
PPAR (peroxisome proliferator-activated receptor) alpha-gamma dual agonist and application thereof
A dual agonist, methyl technology, applied in the field of medicine, can solve the problem of single action
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0117] a) Ethyl 2-methyl-2-(4-acetylphenyl)oxypropionate
[0118] Add 27.2g (0.2mol) of p-hydroxyacetophenone to 250ml of acetonitrile, add 83g (0.6mol) of anhydrous potassium carbonate under stirring, and after stirring for 15min, continue to add 43.0g (0.22mol) of ethyl bromoisobutyrate ), back flow reaction 4h. After the reaction was completed, filter and concentrate the filtrate to obtain a tan oil, add 80 ml of 1 mol / L sodium hydroxide solution, and extract with dichloromethane (80 ml×2). The organic layers were combined, washed with water (50ml×2), and washed with saturated brine (50ml×1). The organic layer was dried over anhydrous sodium sulfate, and the solvent was removed under reduced pressure to obtain 12.3 g of light yellow oily liquid with a yield of 49.2%.
[0119] b) Ethyl 3-{4-[(2-methyl-1-ethoxy-1-oxopropyl)-2-oxyl]phenyl}-3-oxopropionate
[0120] 3.8g (0.096mol) of 60% sodium hydride and 22.7g (0.192mol) of dry diethyl carbonate were dissolved in 100ml of ...
Embodiment 2
[0124] 2-Methyl-2-[4-(6-fluoro-2-oxo-2H-1-benzopyran-3-carbonyl)phenoxy]propanoic acid ethyl ester
[0125] 0.32g (0.001mol) of 3-{4-[(2-methyl-1-ethoxy-1-oxopropyl)-2-oxyl]phenyl}-3-oxopropionic acid ethyl For ester, 0.15g (0.0011mol) of 5-fluorosalicylaldehyde was dissolved in 10ml of absolute ethanol, 5 drops of piperidine and 1 drop of glacial acetic acid were added, and the reaction was refluxed at 80°C for 1h under the protection of nitrogen. After the reaction was completed and cooled to room temperature, a white solid was precipitated, filtered with suction, washed with absolute ethanol, and dried in vacuo to obtain 0.2 g of a white solid with a yield of 50.2%. m.p.101.4~101.9℃. ESI-MS: m / z 399.1[M+H] + ,421.1[M+Na] + ,437.1[M+K] + . 1 H NMR (400MHz, DMSO-d 6 ):δ8.31(s,1H,chromene-4-H),7.91(d,J=8.9Hz,2H,phenyl-H),7.70(dd,J=8.4,3.0Hz,1H,chromene-8- H), 7.61(td, J=8.8Hz, 8.5Hz, 3.0Hz, 1H, chromene-7-H), 7.56(dd, J=9.1, 4.5Hz, 1H, chromene-5-H), 6.86(d ,J=8.9Hz,2H...
Embodiment 3
[0127] 2-Methyl-2-[4-(6-chloro-2-oxo-2H-1-benzopyran-3-carbonyl)phenoxy]propanoic acid ethyl ester
[0128] Using 5-chlorosalicylaldehyde as the raw material, the preparation method was the same as in Example 2 to obtain 0.21 g of white powdery solid with a yield of 50.6%. m.p.100.6~101.0℃. ESI-MS: m / z 415.1[M+H] + ,437.1[M+Na] + . 1 H NMR (400MHz, DMSO-d 6 ): δ8.30(s,1H,chromene-4-H),7.95(d,J=2.5Hz,1H,chromene-5-H),7.91(d,J=8.9Hz,2H,phenyl-H) ,7.76(dd,J=8.9,2.6Hz,1H,chromene-7-H),7.54(d,J=8.9Hz,1H,chromene-8-H),6.86(d,J=8.9Hz,2H, phenyl-H), 4.17(q, J=7.1Hz, 2H, OCH2 ),1.61(s,6H,CH 3 ), 1.15(t, J=7.1Hz, 3H, CH 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com