An antibody-dolastatin conjugate and its preparation method and application
A technology of dolastatin and conjugates, which is applied in the preparation methods of peptides, chemical instruments and methods, anti-animal/human immunoglobulins, etc., can solve the problems of antibody stability or decreased targeting, and achieve uniformity. The effect of good performance and good tumor killing ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Preparation of CLA12 HL-vcMMAE conjugate.
[0037]The monoclonal antibody targeting the EAAGIGILTV / HLA-A2 complex is CLA12, whose sequence comes from US patent: US20100158927A1. Antibody light and heavy chain variable region gene sequence (the heavy chain variable region gene sequence is shown in SEQ ID No.5, and the light chain variable region gene sequence is shown in SEQ ID No.6) from Sangon Bioengineering (Shanghai) Co., Ltd. Co., Ltd. on behalf of the synthesis, the antibody light and heavy chain constant region sequence is preserved in the laboratory, the variable region of the light and heavy chain is connected to the constant region of the human Lamda light chain and the constant region of the human IgG1 type heavy chain by PCR method, and the C-terminal of the light and heavy chain The recombinant antibody obtained by linking the LPETG sequence and expressing it was named CLA12 HL.
[0038] (1) Modification of the C-terminals of the light and heavy chains of t...
Embodiment 2
[0043] Identification of CLA12 HL-vcMMAE conjugates.
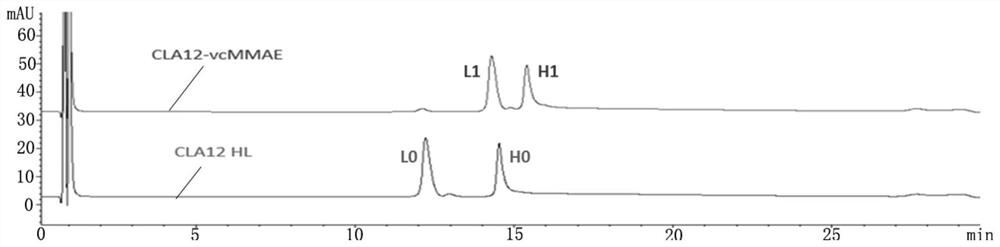
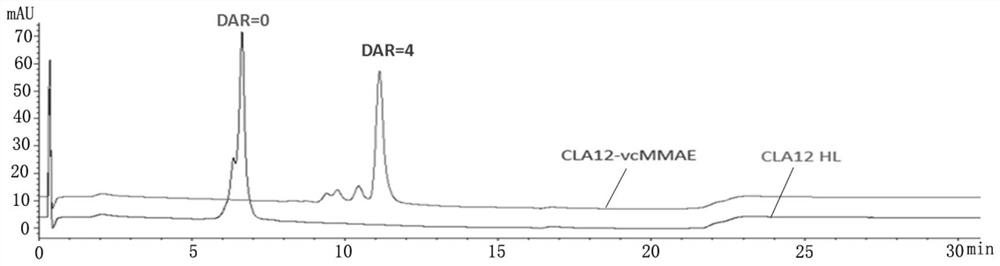
[0044] (1) High performance liquid phase identification of CLA12 HL-vcMMAE conjugated drug, take 20 μg each of CLA12 HL-vcMMAE conjugated drug and CLA12 HL monoclonal antibody, add a little TCEP powder, mix with a pipette gun, and incubate at 37°C for 10 minutes. with PLRP-S The reverse column was used for analysis, and the detection wavelength was 280nm.
[0045] Test results such as figure 1 As shown, CLA12 HL monoclonal antibody has two peaks of light chain (L0) and heavy chain (H0), and CLA12HL-vcMMAE also has two peaks of light chain (L0) and heavy chain (H0), but compared with CLA12 HL monoclonal antibody The positions of the two peaks of the light chain and the heavy chain of the cloned antibody are obviously shifted backwards, and there is no overlap with the positions of the two peaks of the light chain and the heavy chain of the CLA12 HL monoclonal antibody, indicating that the CLA12 HL-vcMMAE light chain and ...
Embodiment 3
[0060] Biological activity detection of CLA12 HL-vcMMAE conjugate.
[0061] 1. Flow affinity detection
[0062] (1) Take 5×10 5 MEL 624.38 cells were incubated with 5 μg / mL CLA12 monoclonal antibody and CLA12 HL-vcMMAE in 1% BSA in PBS solution for 30 min at 4°C;
[0063] (2) After washing twice with PBS, add goat anti-human IgG (H+L) polyclonal antibody (1:200 dilution), and incubate at 4°C for 30 minutes; after washing twice with PBS, detect the average fluorescence intensity of the cells with a shedding cell analyzer (MFI).
[0064] The combination of CLA12 and CLA12 HL-vcMMAE with MART-1, HLA-A2 double-positive cells MEL624.38 was detected by flow cytometry, and the strength of the binding force was shown by the average fluorescence intensity of FITC labeled with the secondary antibody.
[0065] Test results such as Figure 8 As shown, CLA12 monoclonal antibody and CLA12 HL-vcMMAE have almost the same affinity for the same amount of MART-1, HLA-A2 double positive cells...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com