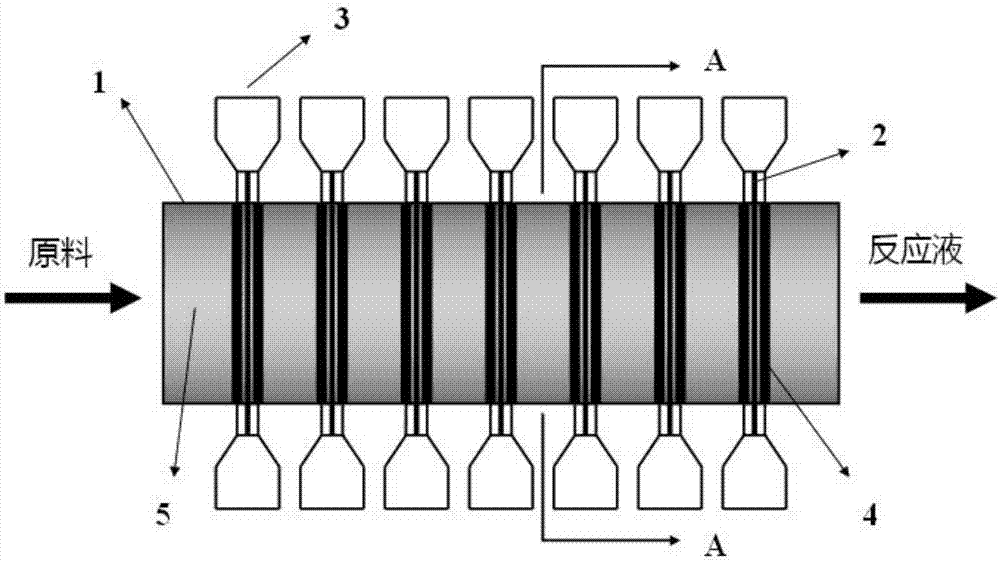
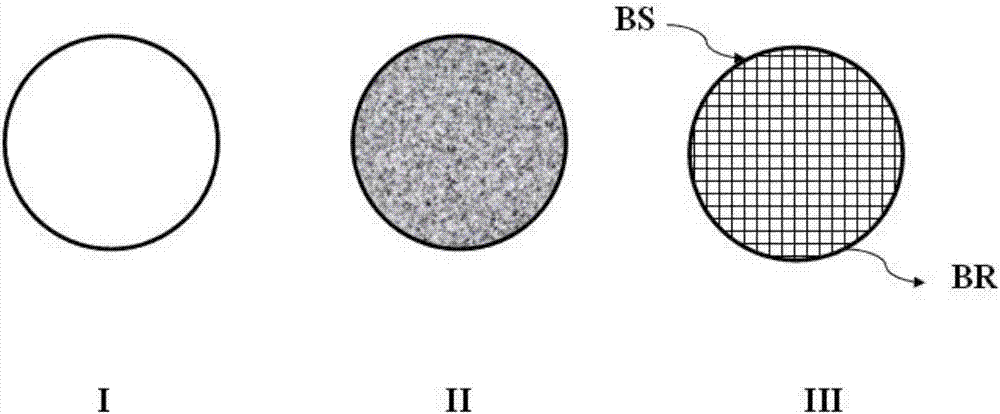
Catalyst, reactor using same and method for preparing beta-phenethyl alcohol
A catalyst and reactor technology, applied in the field of thin-film supported catalysts, can solve problems such as poor catalyst structure and performance, reduced catalyst life, and reduced production efficiency, so as to simplify the product separation process, reduce production costs, and improve production efficiency. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
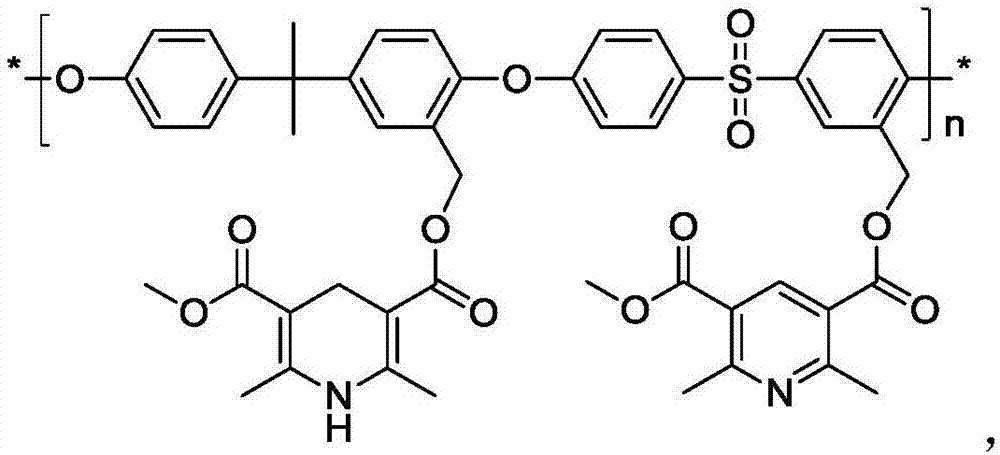
[0048] First, 1,4-dihydropyridine derivatives A and 3-hydroxymethyl-2,2-bis(4-hydroxyphenyl)propane C are used for transesterification to obtain monomer E, and the transesterification conditions are: Take 450gA, 516gC and 2g of p-toluenesulfonic acid into a 2L three-necked flask, and start the reaction by raising the temperature. The reaction temperature is 140°C, the reaction pressure is normal pressure, and the reaction time is 8h. After the reaction, the reaction liquid is rectified to obtain the monomer E .
[0049] Then carry out transesterification with 1,4-dihydropyridine derivatives B and 3-hydroxymethyl-4,4-dichlorodiphenyl sulfone D to obtain monomer F, the transesterification reaction conditions are: weigh 446gB and 951gD Add 2.5g of p-toluenesulfonic acid into a 2L three-necked flask, start the reaction by raising the temperature, the reaction temperature is 180°C, the reaction pressure is normal pressure, and the reaction time is 3h. After the reaction, the reacti...
Embodiment 2
[0053] First, carry out transesterification reaction with 1,4-dihydropyridine derivatives B and 3-hydroxymethyl-2,2-bis(4-hydroxyphenyl)propane C to obtain monomer G, and the transesterification reaction conditions are: Take 446gB, 645gC and 2.2g of p-toluenesulfonic acid into a 2L three-necked flask, heat up to start the reaction, the reaction temperature is 160°C, the reaction pressure is normal pressure, and the reaction time is 5.5h. After the reaction, the reaction solution is rectified to obtain Body G.
[0054] Then 1,4-dihydropyridine derivatives A and 3-hydroxymethyl-4,4-dichlorodiphenyl sulfone D were transesterified to obtain monomer H. The transesterification conditions were: weigh 450gA and 697.4 gD and 2.5g of p-toluenesulfonic acid were added into a 2L three-necked flask, the temperature was raised to start the reaction, the reaction temperature was 175°C, the reaction pressure was normal pressure, and the reaction time was 4.5h. After the reaction, the reaction...
Embodiment 3
[0057] Using monomers E and H as raw materials, the polysulfone microporous membrane-supported 1,4-dihydropyridine Hantzsch ester nicotinamide coenzyme model molecular catalyst CAT-III was prepared referring to the method in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com