Chimeric peptide based on endomorphin-1 and neurotensin (8-13) and synthesis method and application thereof
A technology of neurotensin and endomorphin, applied in the field of chimeric peptides and their synthesis, can solve the problems of low analgesic activity, short analgesic duration, analgesic tolerance and gastrointestinal side effects, and achieve low Side effects, effects of high clinical application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0038] Specific embodiment one: the amino acid sequence of the chimeric peptide based on endomorphin-1 and neurotensin (8-13) in this embodiment is as follows:
[0039] Tyr-Pro-Trp-Phe-Gly-Gly-Arg-Arg-Pro-Tyr-Ile-Leu.
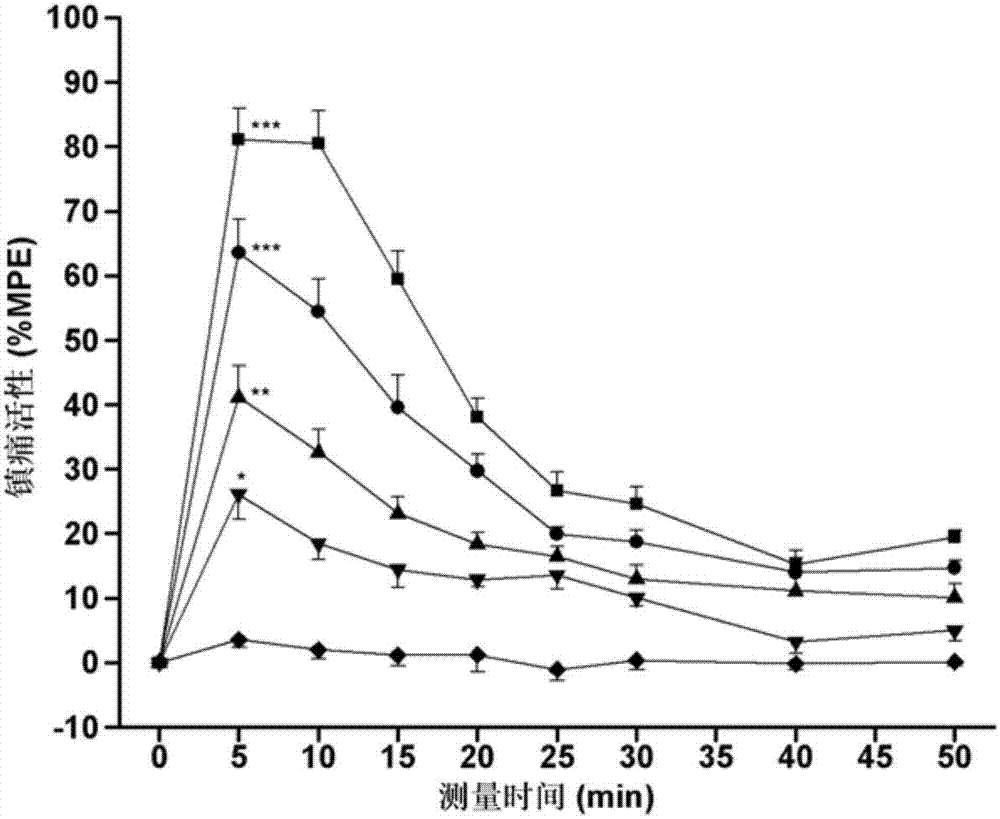
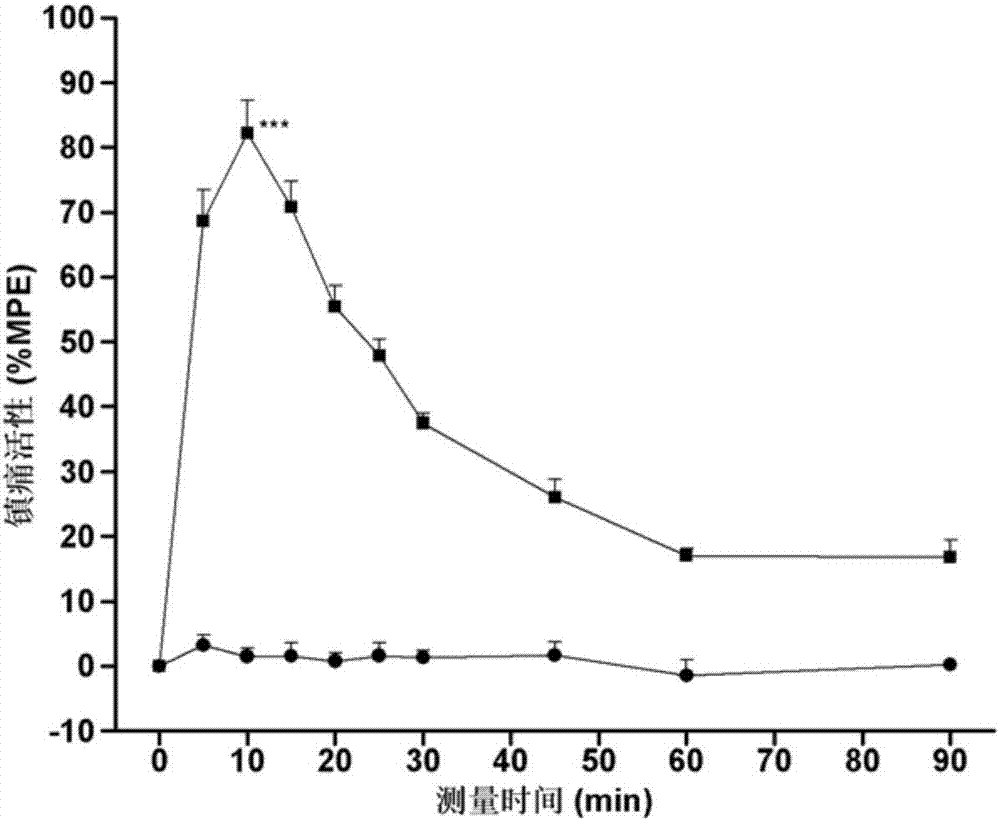
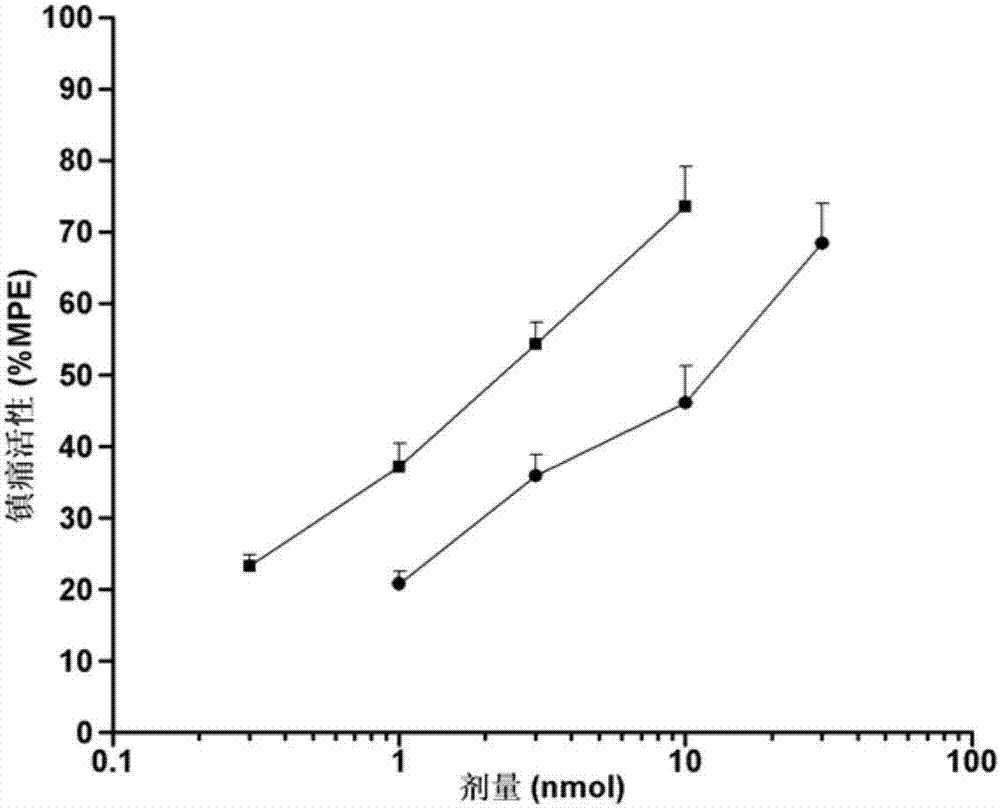
[0040] This embodiment retains the biological activity of the two neuropeptide fragments, and solves the problem of the short analgesic duration of endomorphin-1, the low analgesic activity of peripheral administration, and the analgesic tolerance and gastrointestinal side effects The problem. Through various in vitro and in vivo biological experiments, the pharmacological activity of the chimeric peptide of this embodiment was identified. The results show that the chimeric peptide of this embodiment has higher affinity to μ-opioid receptors and opioid activity on isolated specimens, especially the activity on GPI specimens is higher than that of endomorphin-1. In addition, the chimeric peptide has high analgesic activity of central and peripheral administrat...
specific Embodiment approach 2
[0041] Specific embodiment two: the present embodiment is based on the synthetic method of the chimeric peptide of endomorphin-1 and neurotensin (8-13), comprises the following steps:
[0042] 1. Pretreatment of Wang resin protected by "Fmoc": check the air tightness of the solid phase synthesizer, put the Fmoc-Leu-Wang resin with one amino acid residue into the synthesizer, add dichloromethane and stir for 30-40min, After the resin is fully soaked and swollen, filter the solvent under reduced pressure; the mass ratio of the Fmoc-Leu-Wang resin with one amino acid residue to the volume ratio of dichloromethane is 1g: (7-12)mL;
[0043] 2. Remove the "Fmoc" protecting group: wash the swelled resin with DMF for 3 to 5 minutes, dry it, repeat 3 to 5 times, and then add 20% to 25% vol. Piperidine / DMF deprotection solution, stirred for 5-10 minutes, drained, repeated 2-3 times, then added piperidine / DMF deprotection solution with volume percentage concentration of 20%-25%, stirred ...
specific Embodiment approach 3
[0050] Specific embodiment three: the difference between this embodiment and specific embodiment two is: the molar weight of the amino acid protected by the "Fmoc" group in step three is 2.5-3 times the molar weight of the Fmoc-Arg(pbf)-Wang resin. Others are the same as in the second embodiment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com