Method for preparing aromatic hydrocarbons
A technology for aromatic hydrocarbons and carbon monoxide, applied in chemical instruments and methods, hydrocarbon production from oxygen-containing organic compounds, including molecular sieve catalysts, etc., can solve the problems of rapid decrease in aromatics selectivity, easy sublimation or aggregation of metals, and low BTX selectivity, etc. To achieve the effect of being beneficial to environmental protection, stable selectivity, and simplifying the process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
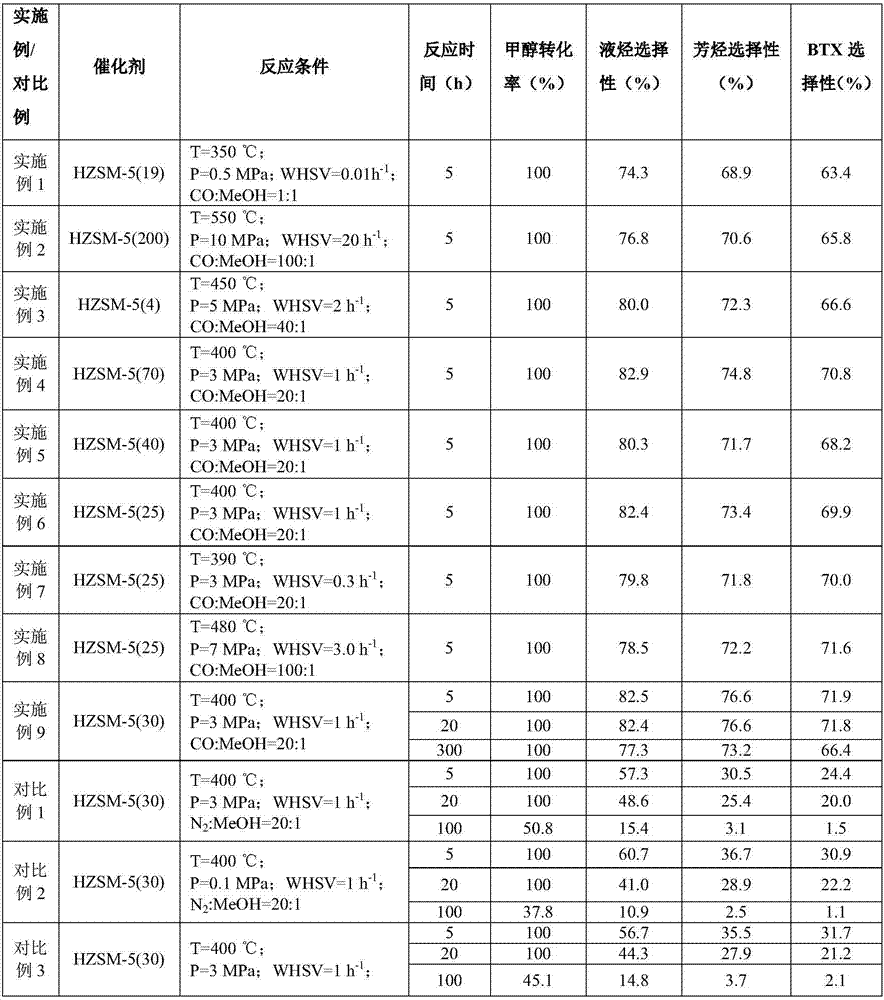
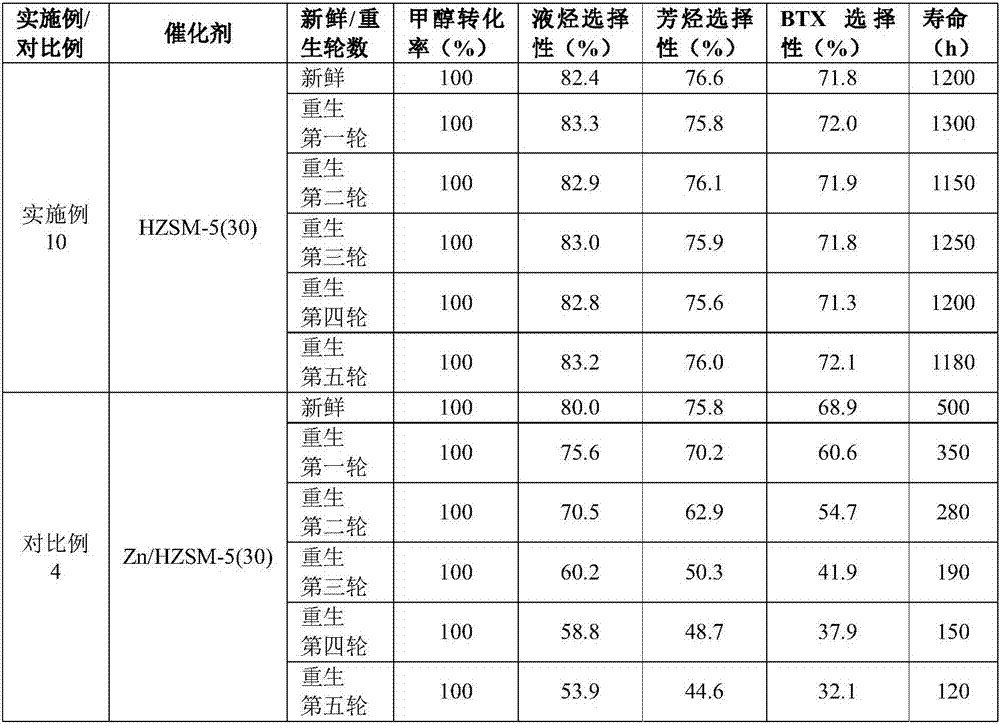
[0035] The 10g Si / Al=19 (atomic ratio) hydrogen-type ZSM-5 molecular sieve purchased by Nankai University Catalyst Factory, referred to as HZSM-5(19), was sieved into 20-40 mesh particles after tableting, and the inner diameter was 16mm. In a stainless steel reaction tube, use 100ml / min nitrogen to activate at 550°C for 4h, and react under the following conditions: reaction temperature (T)=350°C, reaction pressure (P)=0.5MPa, methanol mass space velocity (WHSV)=0.01h -1 , carbon monoxide:methanol (CO:MeOH)=1:1. After the reaction was stable, the product was analyzed by gas chromatography, and the reaction results are shown in Table 1.
Embodiment 2
[0037] 10g of hydrogen-type ZSM-5 molecular sieve with Si / Al=200 (atomic ratio) purchased by Nankai University Catalyst Factory, referred to as HZSM-5(200) for short, was sieved into 20-40 mesh particles after tableting, and the inner diameter was 16mm In a stainless steel reaction tube, use 100ml / min nitrogen to activate at 550°C for 4h, and react under the following conditions: reaction temperature (T)=550°C, reaction pressure (P)=10MPa, methanol mass space velocity (WHSV)=20h -1 , carbon monoxide:methanol (CO:MeOH)=100:1. After the reaction was stable, the product was analyzed by gas chromatography, and the reaction results are shown in Table 1.
Embodiment 3
[0039] The 10g Si / Al=4 (atomic ratio) hydrogen-type ZSM-5 molecular sieve purchased by Shanghai Zhuoyue Co., Ltd., referred to as HZSM-5(4) for short, was sieved into 20-40 mesh particles after tableting, and packed into a 16mm inner diameter In a stainless steel reaction tube, use 100ml / min nitrogen to activate at 550°C for 4h, and react under the following conditions: reaction temperature (T)=450°C, reaction pressure (P)=5MPa, methanol mass space velocity (WHSV)=2h -1 , carbon monoxide:methanol (CO:MeOH)=40:1. After the reaction was stable, the product was analyzed by gas chromatography, and the reaction results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com