Fluorescence probe based on triazole acyl hydrazone derivative and preparation method of fluorescence probe
A technology of triazole acyl hydrazone and fluorescent probe, which is applied in the fields of fluorescence/phosphorescence, chemical instruments and methods, and luminescent materials, and can solve problems such as the toxicity of aluminum ions, achieve strong selectivity, easily obtain raw materials, and realize naked-eye discrimination Detection effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
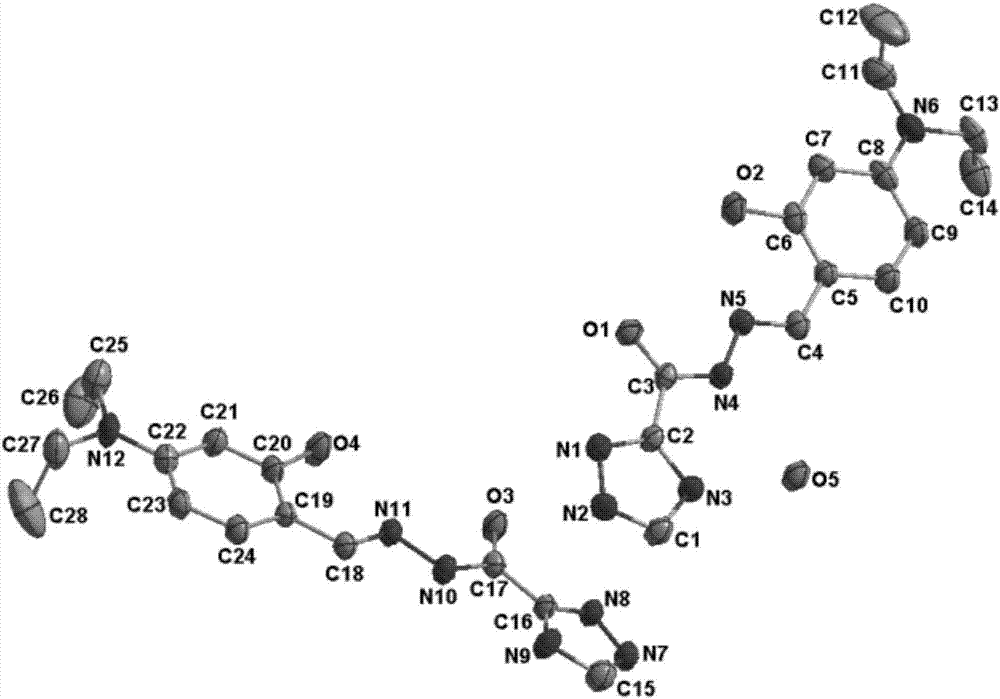
[0030] The synthesis diagram of triazole acylhydrazone derivatives is shown in figure 1 .
[0031] Dissolve 1.93g of 4-diethylamino salicylaldehyde in 30mL of absolute ethanol, then add 1.27g of 1,2,4-triazole-3-carboxylhydrazide, reflux and stir for 2 hours under normal pressure, and precipitate a large amount of The solid was filtered under reduced pressure, and the filter residue was washed with absolute ethanol to obtain a light yellow solid, which was the target product, and the yield of the target product was 79%.
[0032] The crystal structure of triazole acylhydrazone derivative hydrate is shown in figure 1 ;
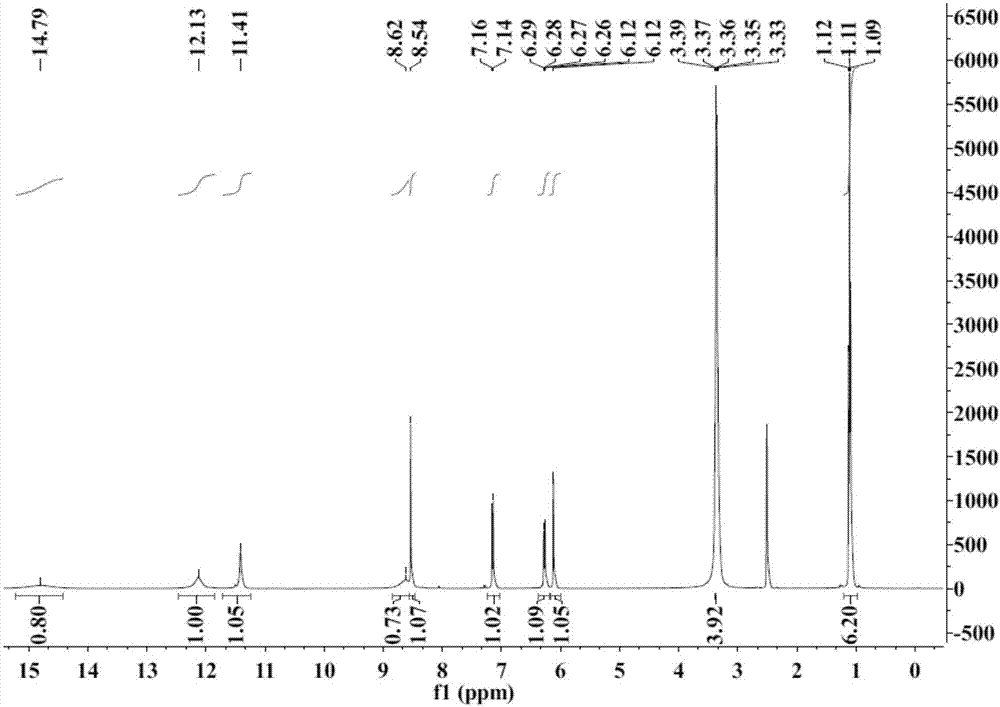
[0033] Adopt nuclear magnetic resonance instrument to carry out nuclear magnetic resonance analysis to the prepared triazole acyl hydrazone derivatives, the results are as follows:
[0034] 1 H NMR (400MHz, DMSO-d 6 ),δ(ppm):14.79(s,1H,OH),12.13(s,1H,NH),11.41(s,1H,NH-triazole),8.62(s,1H,CH-triazole),8.54(s ,1H,CH=N),7.14-7.16(d,1H,Aryl-H),6.26-6.29(dd,1H,...
Embodiment 2
[0037] Dissolve 3.86g of 4-diethylamino salicylaldehyde in 80mL of 75% ethanol, then add 2.54g of 1,2,4-triazole-3-carboxylhydrazide, reflux and stir for 4 hours under normal pressure, and precipitate a large amount of solid after cooling to room temperature , filtered under reduced pressure, and the filter residue was washed with 75% ethanol to obtain a light yellow solid, which was the target product, and the yield of the target product was 85%.
Embodiment 3
[0039] Dissolve 3.86g of 4-diethylamino salicylaldehyde in 80mL of absolute ethanol, then add 2.54g of 1,2,4-triazole-3-carboxylhydrazide, reflux and stir for 3 hours under normal pressure, and precipitate a large amount of The solid was filtered under reduced pressure, and the filter residue was washed with absolute ethanol to obtain a pale yellow solid, which was the target product, and the yield of the target product was 82%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com