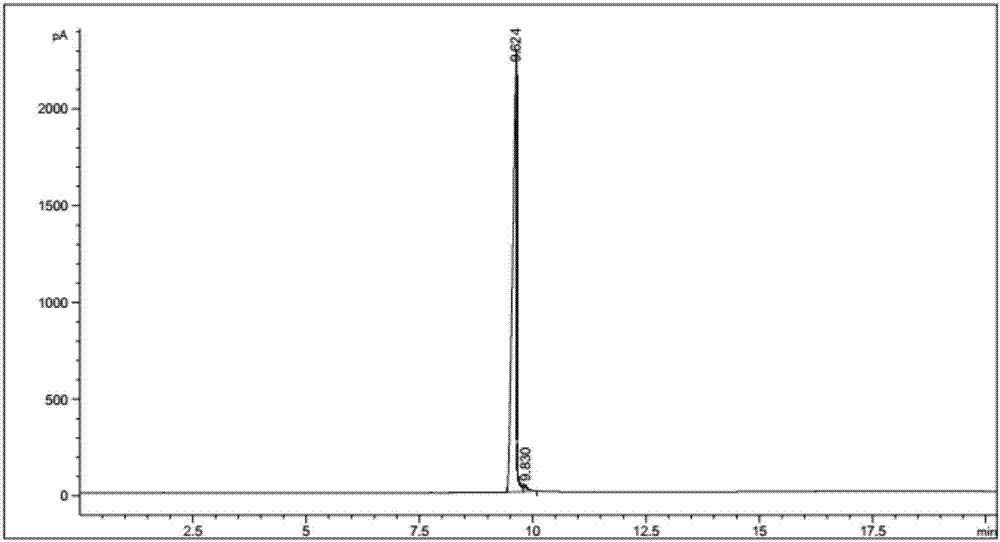
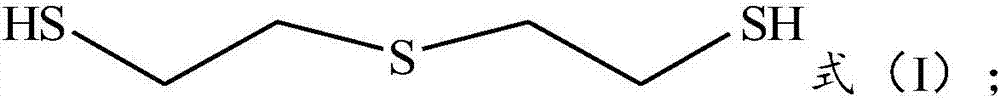
Preparation method of thiodiethanethiol
A technology of thiodiglycol and thiodiglycol, applied in thioether preparation, organic chemistry and other directions, can solve the problems of low product quality, difficult treatment, large amount of waste water, etc., to maximize resource value, The effect of maximizing comprehensive utilization and low energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0028] The invention provides a kind of preparation method of thiodiglycolyl mercaptan, comprises the following steps:
[0029] a) mixing thiodiglycol, thiourea and hydrochloric acid to perform a salt-forming reaction, and then adding an alkali solution to perform a hydrolysis reaction to obtain a reaction mixture;
[0030] b) Separating the reaction mixture obtained in step a) to obtain the crude thiodiglycol mercaptan and reaction waste water respectively; waste water;
[0031] c) performing flocculation treatment on the reaction waste water and washing waste water obtained in step b), respectively, to obtain recycled water.
[0032] In the invention, the thiodiglycol, thiourea and hydrochloric acid are firstly mixed for a salt-forming reaction, and then an alkali solution is added for a hydrolysis reaction to obtain a reaction mixture. In the present invention, the hydrochloric acid is preferably concentrated hydrochloric acid with a mass concentration of 37%. In the pre...
Embodiment 1
[0059] (1) The preparation of isothiourea hydrochloride: add 34g thiourea in the 250mL four-neck flask that thermometer, agitator, ventilation device are housed, start stirring, then add 34g mass concentration and be the concentrated hydrochloric acid of 37%, be warming up to Dissolve, then directly add 25g of thiodiethylene glycol, and heat up to reflux after adding; keep warm after reflux, take samples every 2h to follow up, if there is raw material thiodiethylene glycol, continue the heat preservation reaction, if there is no thiodiethylene glycol, then Stop the reaction, cool down;
[0060] (2) Hydrolysis of isothiourea hydrochloride: lower the temperature to below 60°C, add dropwise 300mL of 10% sodium hydroxide solution into the flask, then raise the temperature to 70°C, keep warm for 3h, and the reaction is completed to obtain a reaction mixture.
[0061] (3) Pour the reaction mixture obtained in step (2) into a separatory funnel and let stand for 30min to separate liqu...
Embodiment 2
[0066] (1) The preparation of isothiourea hydrochloride: add 35g thiourea in the 250mL four-neck flask that thermometer, agitator, ventilation device are housed, start stirring, then add 35g mass concentration and be the concentrated hydrochloric acid of 37%, be warming up to Dissolve, then directly add 25g of thiodiethylene glycol, and heat up to reflux after adding; keep warm after reflux, take samples every 2h to follow up, if there is raw material thiodiethylene glycol, continue the heat preservation reaction, if there is no thiodiethylene glycol, then Stop the reaction, cool down;
[0067] (2) Hydrolysis of isothiourea hydrochloride: lower the temperature to below 60°C, add dropwise 300mL of 10% sodium hydroxide solution into the flask, then raise the temperature to 70°C, keep warm for 3h, and the reaction is completed to obtain a reaction mixture.
[0068](3) Pour the reaction mixture obtained in step (2) into a separatory funnel and let stand for 30min to separate liqui...
PUM
| Property | Measurement | Unit |
|---|---|---|
| refractive index | aaaaa | aaaaa |
| refractive index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com