Salmonella Indiana PCR (polymerase chain reaction) detection kit and non-diagnosis detection method thereof
A detection kit and technology for Salmonella, applied in the field of molecular biology detection, can solve the problems such as the reports on PCR detection methods and kits for Salmonella Indiana serotypes that have not yet been found, and achieve detection cost advantages, strong specificity, and detection time advantages. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] The establishment of embodiment 1 Salmonella Indiana PCR detection method
[0024] Design of primers: According to the genomic DNA sequences of different serotypes of Salmonella known in the GenBank database, after comparative genomics analysis and comparison, the specific gene fragments of Salmonella Indiana were screened out, and on this basis, they were designed and optimized for specific The PCR primers for detection of Salmonella Indiana are listed in the table below.
[0025] Table 1 Primer sequences for PCR detection of Salmonella Indiana
[0026]
[0027] Preparation of PCR template: Salmonella Indiana ATCC51959 was cultured in Brain Heart Infusion Broth Medium, and bacterial genomic DNA was extracted using a commercial bacterial genomic DNA extraction kit as a template to be tested.
[0028] Preparation of PCR detection reagent: 10× PCR buffer (its components include 100mM KCl, 80mM (NH4) SO4, pH 9.0 of 100mM Tris-HCl, 15mM MgCl2 and 0.5% Tergitol-type NP-4...
Embodiment 2
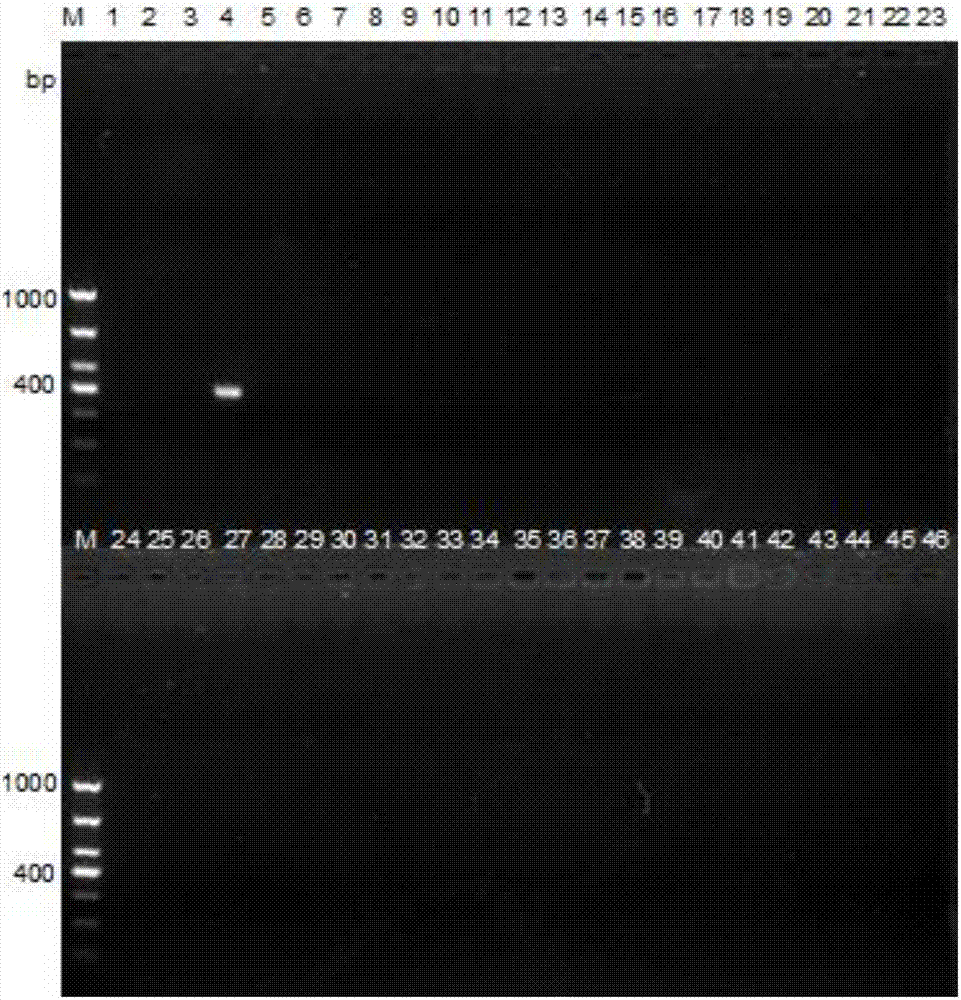
[0032] Embodiment 2 specificity evaluation experiment
[0033] Carry out the specificity evaluation of the PCR detection method of the present invention with the method for Example 1, the test strain is enriched and cultivated in the brain-heart infusion broth medium, and the bacterial genomic DNA is extracted using a commercialized bacterial genomic DNA extraction kit, Carry out PCR detection according to the method described in embodiment 1, the result is as follows figure 2 As shown, only Salmonella Indiana (swimming lane 4) showed a specific amplification band, and other Salmonella serotypes and non-Salmonella strains had no specific amplification. The strains used and the test results are shown in the table below. In the table, "+" in the test result column means positive; "-" means negative.
[0034] Table 2 The specificity evaluation test result of the present invention
[0035]
[0036]
[0037] Since the PCR amplified sequence of the present invention contains ...
Embodiment 3
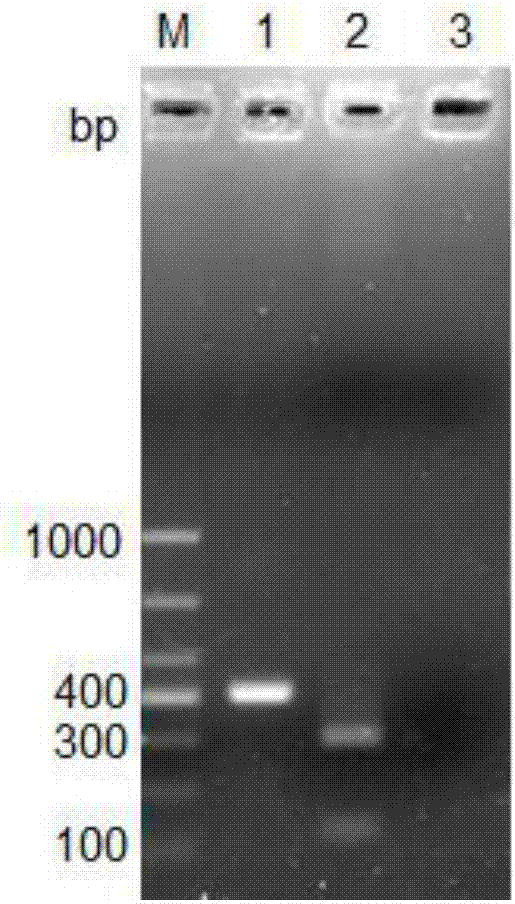
[0038] Embodiment 3 Sensitivity evaluation experiment
[0039] The method of Example 1 was used to evaluate the sensitivity of the PCR detection method of the present invention. Salmonella Indiana ATCC51959 was cultured in Brain Heart Infusion Broth Medium, and the bacterial genome was extracted using a commercially available bacterial genomic DNA extraction kit. After measuring the original concentration, the bacterial genomic DNA was diluted 10 times. Carry out PCR amplification; at the same time, the bacterial solution is subjected to 10-fold gradient dilution after bacterial counting, and bacteria of different concentrations are taken for PCR amplification (see Table 3 for the PCR detection reaction system). After PCR amplification, 5 μl of the amplification product was taken, mixed with 1 μl of 6×Loading buffer, and detected by 2% agarose gel electrophoresis. Electrophoresis results such as Figure 4 As shown, M is DL-1000 marker, and swimming lanes 1-6 are the results ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com