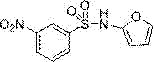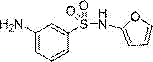Sulfonamide derivative and application thereof in osteoporosis drugs
A technology of derivatives and sulfonamide, applied in the field of sulfonamide derivatives and their application in osteoporosis drugs, can solve problems such as heavy economic burden on patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] : Embodiment 1: the synthesis of N-(furan-2-yl)-3-nitrobenzenesulfonamide:
[0026]
[0027] 3-Nitrobenzenesulfonyl chloride (1.22 mmol) was slowly added dropwise to a solution of furan-2-amine (0.812 mmol) in pyridine (10 mL) previously cooled in an ice-water bath. The mixture was stirred at 0 °C overnight. use CH 2 Cl 2 (20 mL) and extract the mixture with 3N HCl. use CH 2 Cl 2 The aqueous part was extracted once, then washed with Na 2 SO 4 The organic layer was dried and concentrated. The mixture was purified by column chromatography using ethyl acetate and hexane to afford N-(furan-2-yl)-3-nitrobenzenesulfonamide with a purity of 99%. Alternatively add water (100ml) to collect the solid and remove from MeOH:CH 2 Cl 2 Medium recrystallization gave 0.190 g of white crystals with a yield of 87%. 1 H-NMR (400 MHz, CDCl3) δ:6.67(t,1H), 6.99(dd, 1H), 7.87(dd, 1H), 7.96(t,1H), 8.42(s, 1H), 8.53(d, 1H), 8.88(s, 1H). 13 C-NMR (125 MHz, CDCl3) δ:103.06,108.09,...
Embodiment 2
[0028] Example 2: Synthesis of 3-amino-N-(furan-2-yl)benzenesulfonamide:
[0029]
[0030] A Pd-C (5 mmol) catalyst was added to a solution of N-(furan-2-yl)-3-nitrobenzenesulfonamide (10 mmol) in ethanol. In the presence of hydrazine hydrate (50 mmol), the mixture was reacted at 100°C under 150W microwave irradiation for 20 minutes. The progress of the reaction was monitored by TLC. After the reaction was complete the catalyst was isolated by filtration. The reaction solvent was evaporated under reduced pressure to obtain 2.18 g of a liquid product, 3-amino-N-(furan-2-yl)benzenesulfonamide, with a yield of 92%. 1 H-NMR (400 MHz, CDCl3) δ:3.61(s,2H), 6.66(t, 1H), 6.87(d, 1H), 6.98(d, 1H), 7.06(s, 1H), 7.27(d, 1H), 7.35(t,1H), 7.86(d, 1H). 13 C-NMR (125 MHz, CDCl3) δ:103.06, 108.09, 114.70, 121.15,124.39, 126.99, 137.34, 141.60, 145.61,160.02. LC-MS(ESI, pos, ion) m / z: 239[M+1 ].
Embodiment 3
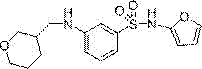
[0031] Example 3: Synthesis of N-(furan-2-yl)-3-(((tetrahydro-2H-pyran-3-yl)methyl)amino)benzenesulfonamide
[0032]
[0033]In a glass flask with a capacity of 200 ml equipped with a stirring device, a thermometer, a reflux condenser and a dropping funnel, 11.39 g (47.8 mmol) of 3-amino-N-(furan-2-yl) benzenesulfonamide, 6.93 g of potassium carbonate g (50.1 mmol) and 60 ml of N,N-dimethylformamide were added under nitrogen atmosphere. While stirring at room temperature, 8.99 g (50.2 mmol) of 3-methyl bromide-tetrahydropyran was added, and the mixture was reacted at 70-80°C for 3-4 hours. After the reaction was completed, the mixture was cooled to room temperature, and then 200 ml of toluene was added. After washing twice with water (180 ml), it was dried over magnesium sulfate. After filtration, it was concentrated under reduced pressure. The obtained yellow oil was then purified using silica gel column chromatography (filler: Wakogel C-200, eluent: hexane / ethyl acetat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com