A kind of pharmaceutical composition containing carbamazepine and biotin and application thereof
A composition and biotin technology, applied in the field of medicine, to achieve the effect of reducing mortality, good safety and significant therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
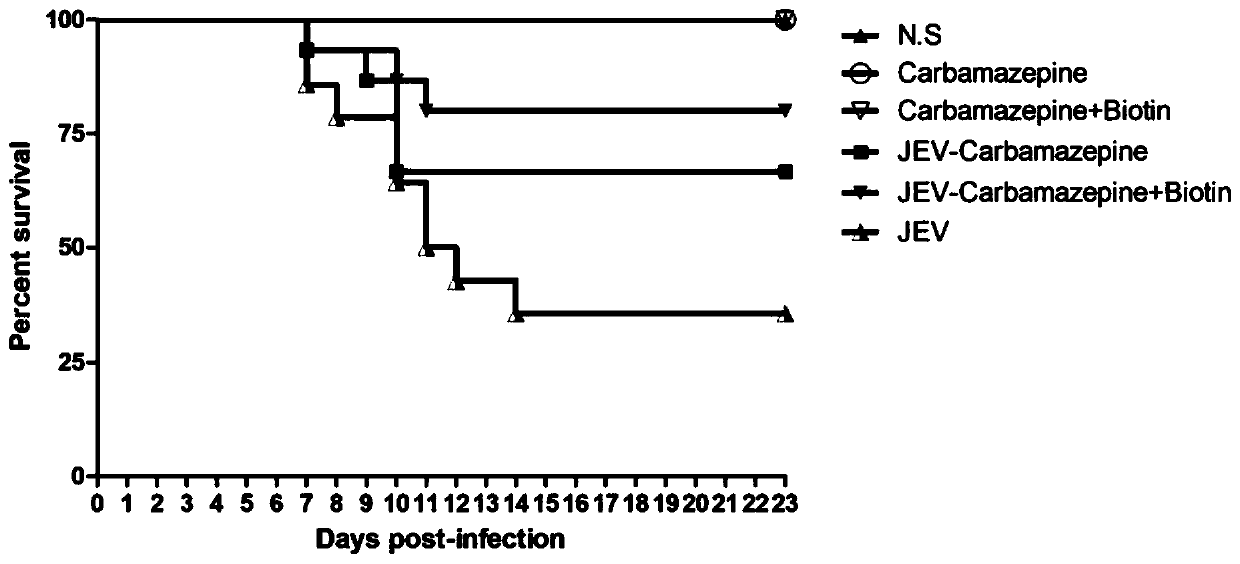
[0036] Example 1 Application of carbamazepine combined with biotin in treating mice infected with Japanese encephalitis virus
[0037] 1. Establishment of a mouse model infected with Japanese encephalitis virus
[0038] 1. Experimental materials:
[0039] (1) Experimental animals: four-week-old Balb / c female mice.
[0040] (2) Strain: JEV P3 strain.
[0041] (3) Other reagents:
[0042] 1) 0.01M PBS buffer: weigh NaH 2 PO4 0.593g, Na 2 HPO4 5.802g, NaCl17.0g, deionized water (ddH 2 O) Dilute to 2L, store at room temperature for later use.
[0043] 2) DMEM basal medium: a bottle of DMEM powder, weighed NaHCO 3 3.7g, HEPES5.95g, add 800mLddH 2 O, stir well to dissolve, dilute to 1000mL, sterilize with a 0.22μm filter, and store at 4°C for later use.
[0044] 2. Experimental steps
[0045] Mice were injected intraperitoneally with 10 6 PFU Japanese encephalitis virus, the mice in the control group were intraperitoneally injected with the same dose of DMEM medium. In ...
Embodiment 2
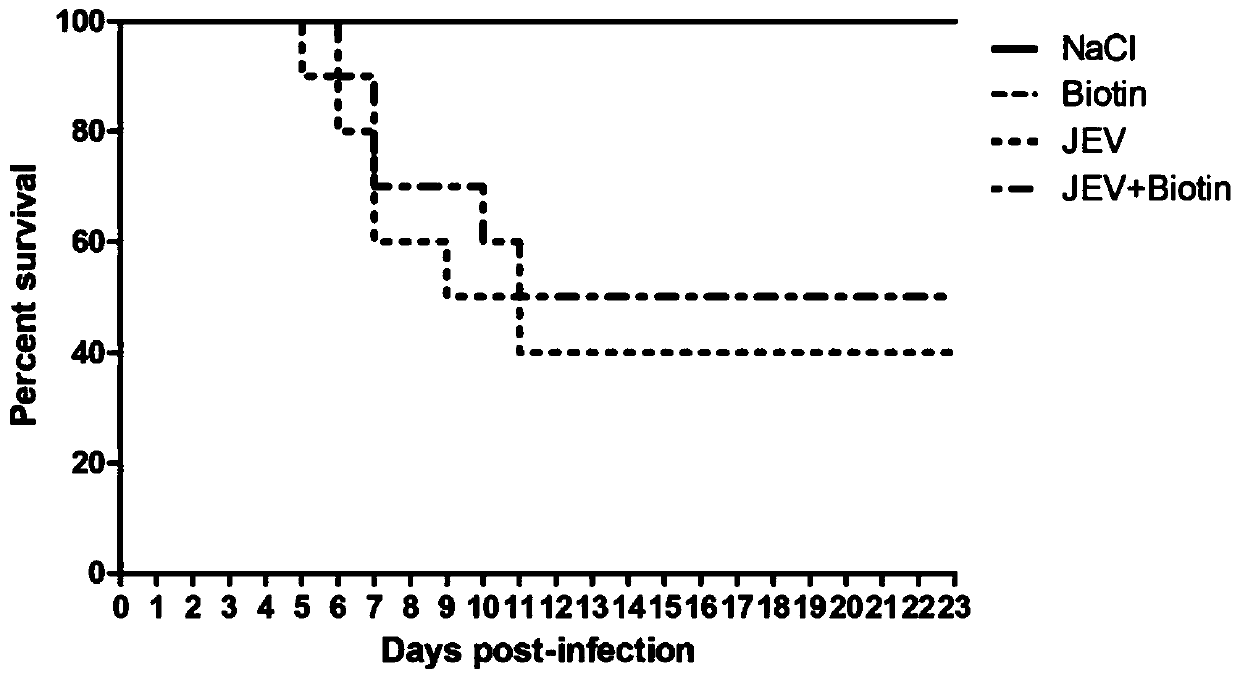
[0064] Example 2 Application of biotin in treating mice infected with Japanese encephalitis virus
[0065] 1. Establishment of a mouse model infected with Japanese encephalitis virus
[0066] Step is with embodiment 1
[0067] 2. Evaluation of the therapeutic effect of biotin on mice infected with Japanese encephalitis virus
[0068] 1. Experimental drugs
[0069] Biotin, traits: white powder, usage: 5% DMSO+30% PEG+ddH 2 O dissolved and administered orally.
[0070] 2. Specific experimental steps
[0071] The content of this part is consistent with the experimental procedure in Example 1, and the oral dose of biotin is 3 mg / kg. Observe and record the number of dead mice.
[0072] 3. Experimental results
[0073] 1. The effect of biotin on the survival rate of JEV-infected mice.
[0074] The challenge experiment lasted until 23 days after infection, and the surviving mice basically had no obvious neurological symptoms. In the 5-10 days of infection, the number of deat...
Embodiment 3
[0078] Example 3 Drug efficacy prediction experiment based on flavivirus infection personalized drug method
[0079] (1) Obtain the gene expression data of Japanese encephalitis virus-infected patients, West Nile virus-infected patients and healthy people.
[0080] The chip data (series: GSE57647, GSE43190) of Japanese encephalitis virus patients, West Nile virus infected patients and control group were downloaded from NCBI GEO Dataset, and a total of 6 Japanese encephalitis virus infected patients and 18 infected patients were obtained. Gene expression data from West Nile virus patients.
[0081] (2) Calculating the key gene list of patients with flavivirus infection
[0082] Based on the gene names in the 24 chip data downloaded in (1) and the gene interaction information downloaded from the STRING database, construct a gene interaction matrix, and calculate the multiple difference value of each gene in each patient as the initial importance input into the GeneRank algorith...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com