Method for removing fluorine ions from F22 by-product hydrochloric acid
A technology for by-product hydrochloric acid and fluoride ions, which is applied to chemical instruments and methods, halogen/halogen acids, compounds of elements in Group 4/14 of the periodic table, etc., can solve the problem of high fluoride ion content, and achieve simple and reliable process equipment. Strong operability and achieve the effect of repeated use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
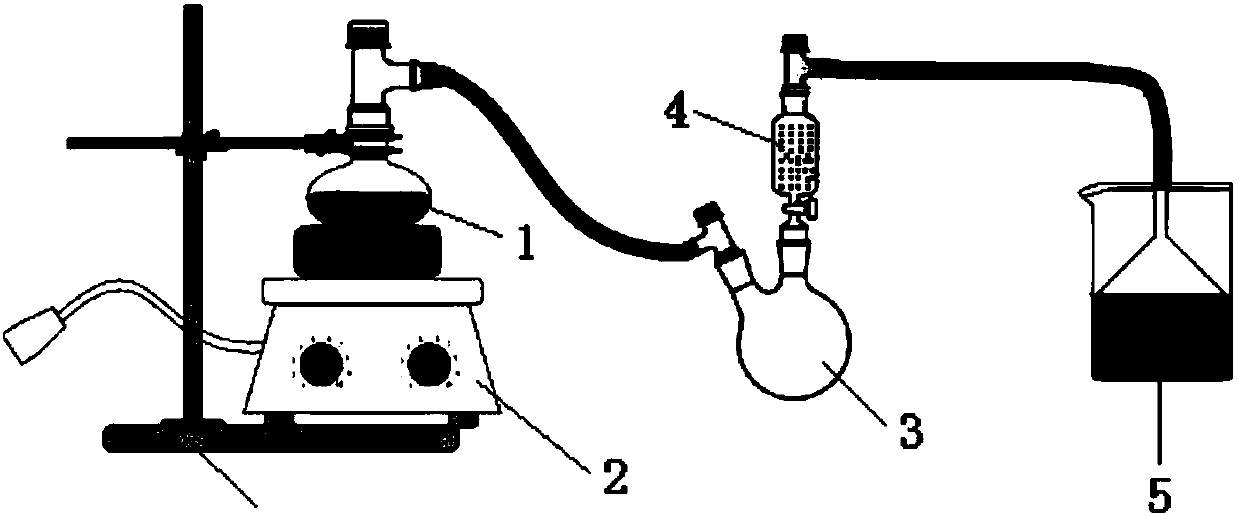
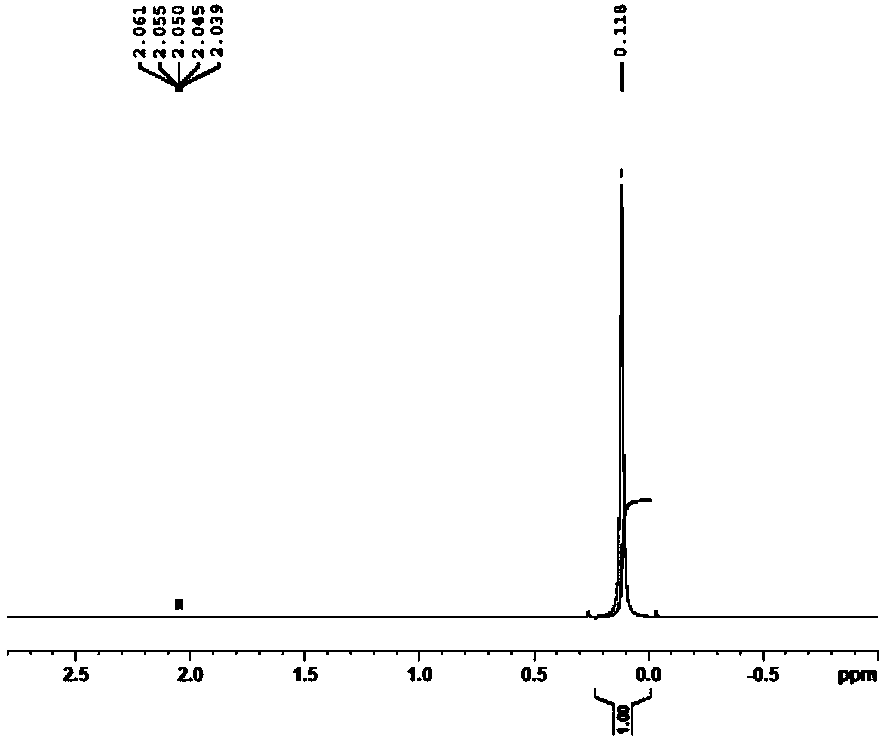
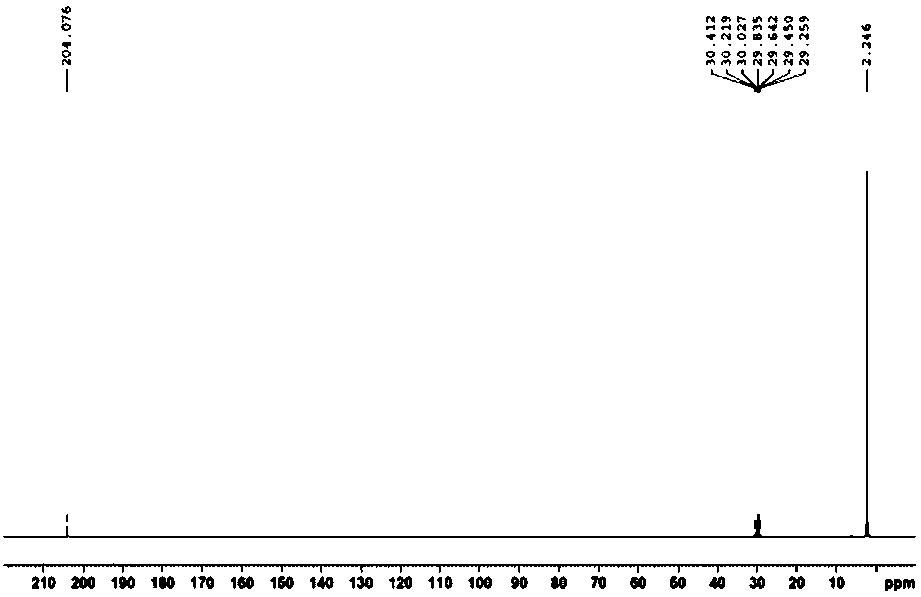
[0048] This example provides a method for removing F 22 The method for fluoride ions in by-product hydrochloric acid, the method is specifically: weigh 15.4g of hexamethyldisiloxane and 180mL of by-product hydrochloric acid in a single-necked flask, the content of the original fluoride ion of hydrochloric acid is 1030.9ppm, hexamethyldisiloxane The actual amount of ether for fluoride ions is 20 times the theoretical amount, sealed to isolate the air, kept at a constant temperature of 30°C, and stirred at high speed by a magnetic stirrer for 2 hours. The drying tower uses granular calcium oxide, and 0.1mol / L NaOH is used to recover the solution. After finishing, let the reaction solution stand still, that is, the fluoride ion is removed.
[0049] The present embodiment adopts the following method to measure the fluoride ion content: accurately measure 10mL sample with a clean pipette, put it in a 50mL beaker, add 10mL total ionic strength adjustment buffer, and mix it with 100g...
Embodiment 2
[0051] This example provides a method for removing F 22 The method for fluoride ions in by-product hydrochloric acid, the method is specifically: weigh 15.4g of hexamethyldisiloxane and 180mL of by-product hydrochloric acid in a single-necked flask, the content of the original fluoride ion of hydrochloric acid is 1417.7ppm, hexamethyldisiloxane The actual amount of ether for fluoride ions is 15 times the theoretical amount, sealed to isolate the air, kept at a constant temperature of 30°C, and stirred at high speed by a magnetic stirrer for 2 hours. The drying tower uses granular calcium oxide, and 0.1mol / L NaOH is used to recover the solution. After finishing, let the reaction solution stand still, that is, the fluoride ion is removed.
[0052] The present embodiment adopts the following method to measure the fluoride ion content: accurately measure 10mL sample with a clean pipette, put it in a 50mL beaker, add 10mL total ionic strength adjustment buffer, and mix it with 100g...
Embodiment 3
[0054] This example provides a method for removing F 22 The method for fluoride ions in by-product hydrochloric acid, the method is specifically: weigh 9.0g of hexamethyldisiloxane and 450mL of by-product hydrochloric acid in a single-necked flask, the content of the original fluoride ion of hydrochloric acid is 492.4ppm, hexamethyldisiloxane The actual amount of ether for fluoride ions is 10 times the theoretical amount, sealed to isolate the air, kept at a constant temperature of 30°C, and stirred at a high speed by a magnetic stirrer for 1 hour. The drying tower uses granular calcium oxide, and 0.1mol / L NaOH is used to recover the solution. After finishing, let the reaction solution stand still, that is, the fluoride ion is removed.
[0055] The present embodiment adopts the following method to measure the fluoride ion content: accurately measure 10mL sample with a clean pipette, put it in a 50mL beaker, add 20mL total ionic strength adjustment buffer, and mix it with 200g / ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com