Preparation method of osimertinib mesylate process impurity
A methoxyl and methylamino technology, applied in the field of drug synthesis, can solve the problems of impurity reporting and process impurity non-reporting, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
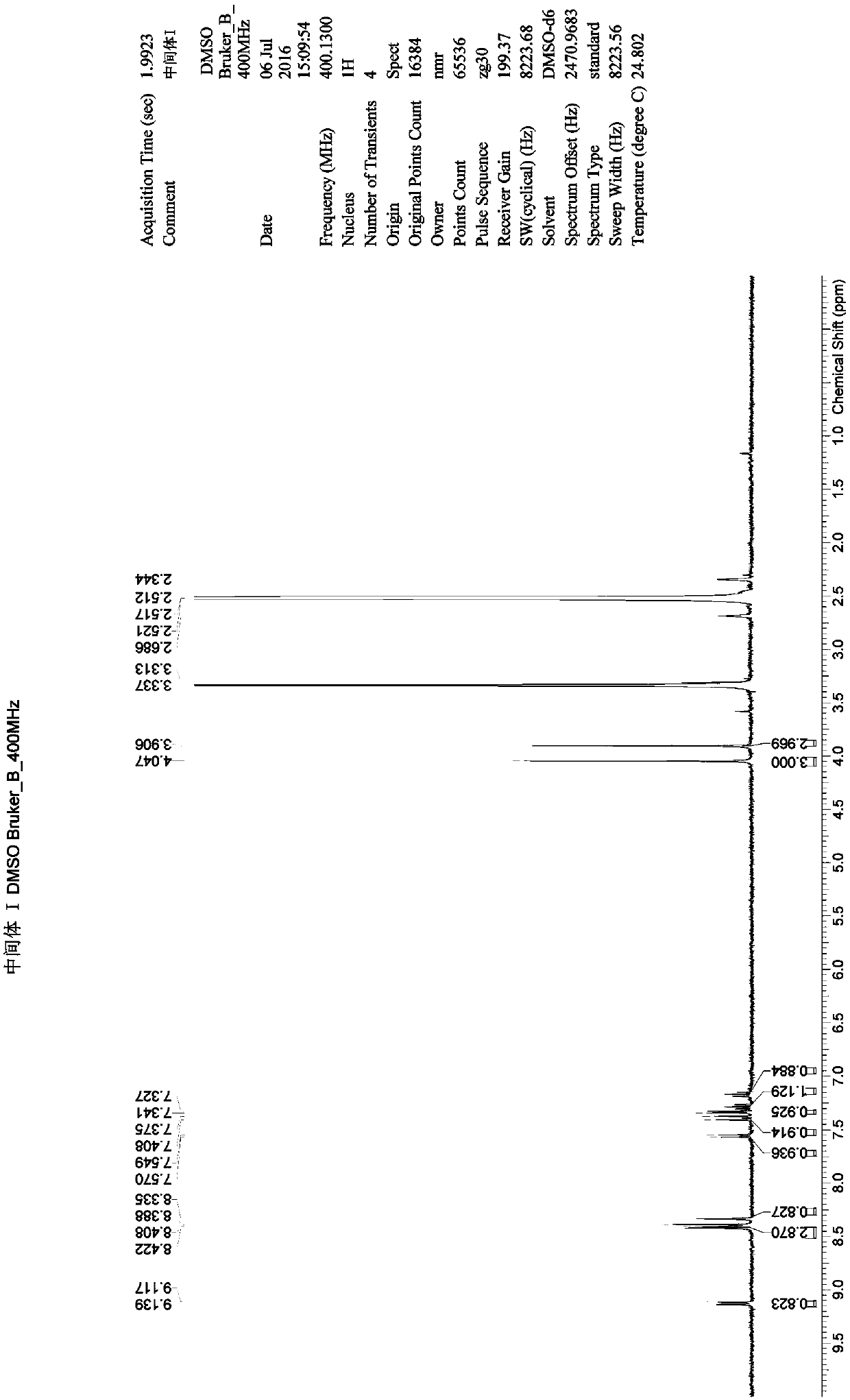
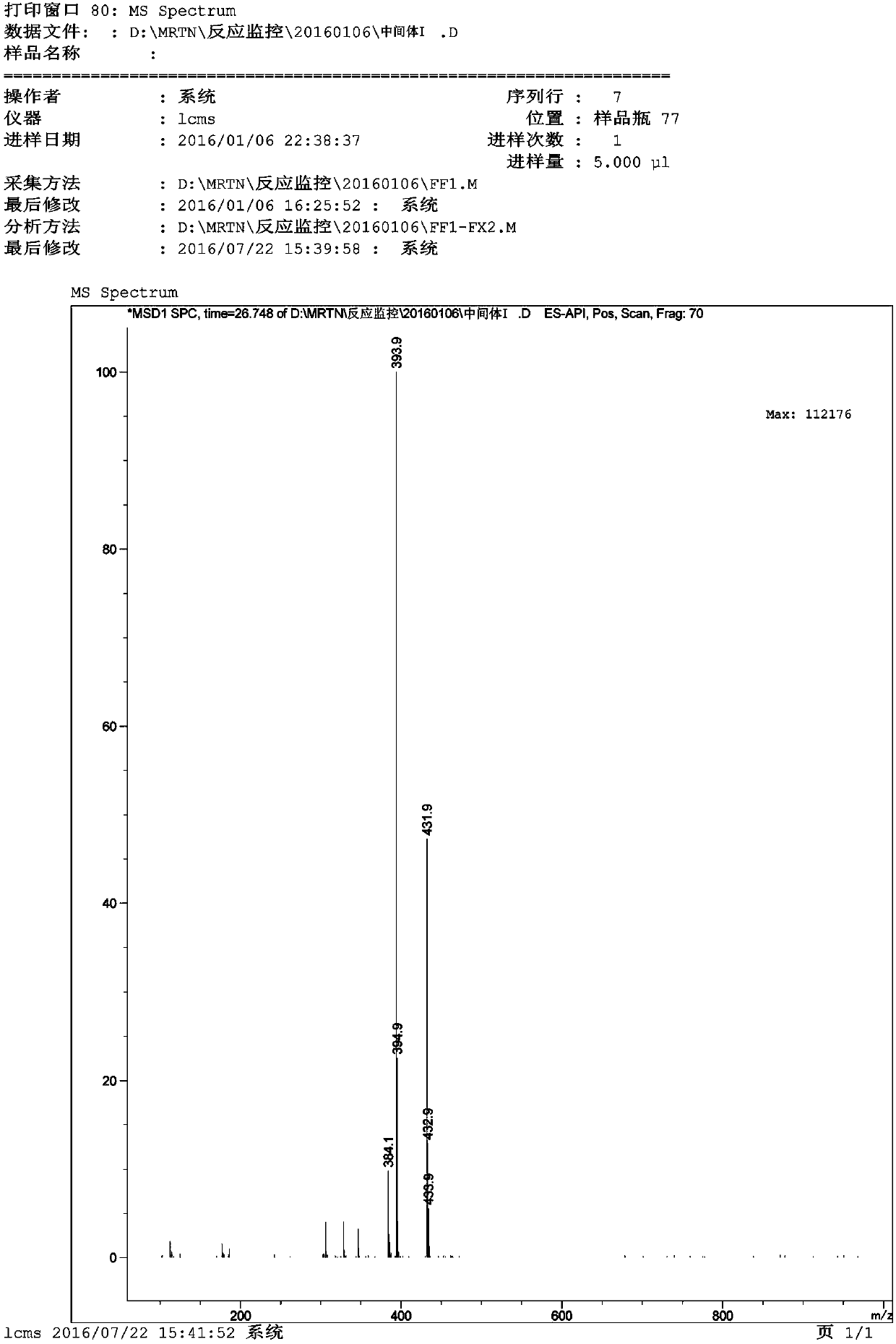
[0052] Example 1: Preparation of N-(4-fluoro-2-methoxy-5-nitro-phenyl)-4-(1-methylindol-3-yl)-pyrimidin-2-amine (MRTN -1)
[0053] Add 1,4-dioxane (2.06L) into the 5L reaction flask, and add 3-(2-chloropyrimidin-4-yl)-1-methylindole (SM1) (175.90g) in sequence under stirring 4-Fluoro-2-methoxy-5-nitroaniline (SM2) (161.20g) and p-toluenesulfonic acid (164.80g) were heated up to 85-90°C, and kept at this temperature for 5h. Cool down to room temperature, add a mixture of 25% ammonia (147.50 g) and water (686.00 mL) dropwise, keep the temperature not exceeding 20° C., and stir for 2 h after dropping. After suction filtration, the filter cake was rinsed with 1,4-dioxane to obtain the crude intermediate I. Add the crude intermediate I to a 5L three-necked flask, add dichloromethane (2.00L), stir and beat for 2 hours below 20°C. Suction filtration, rinse the filter cake with dichloromethane, and blow-dry the material at 45°C-50°C for 6h-10h to obtain a yellow-brown solid (260.00...
Embodiment 2
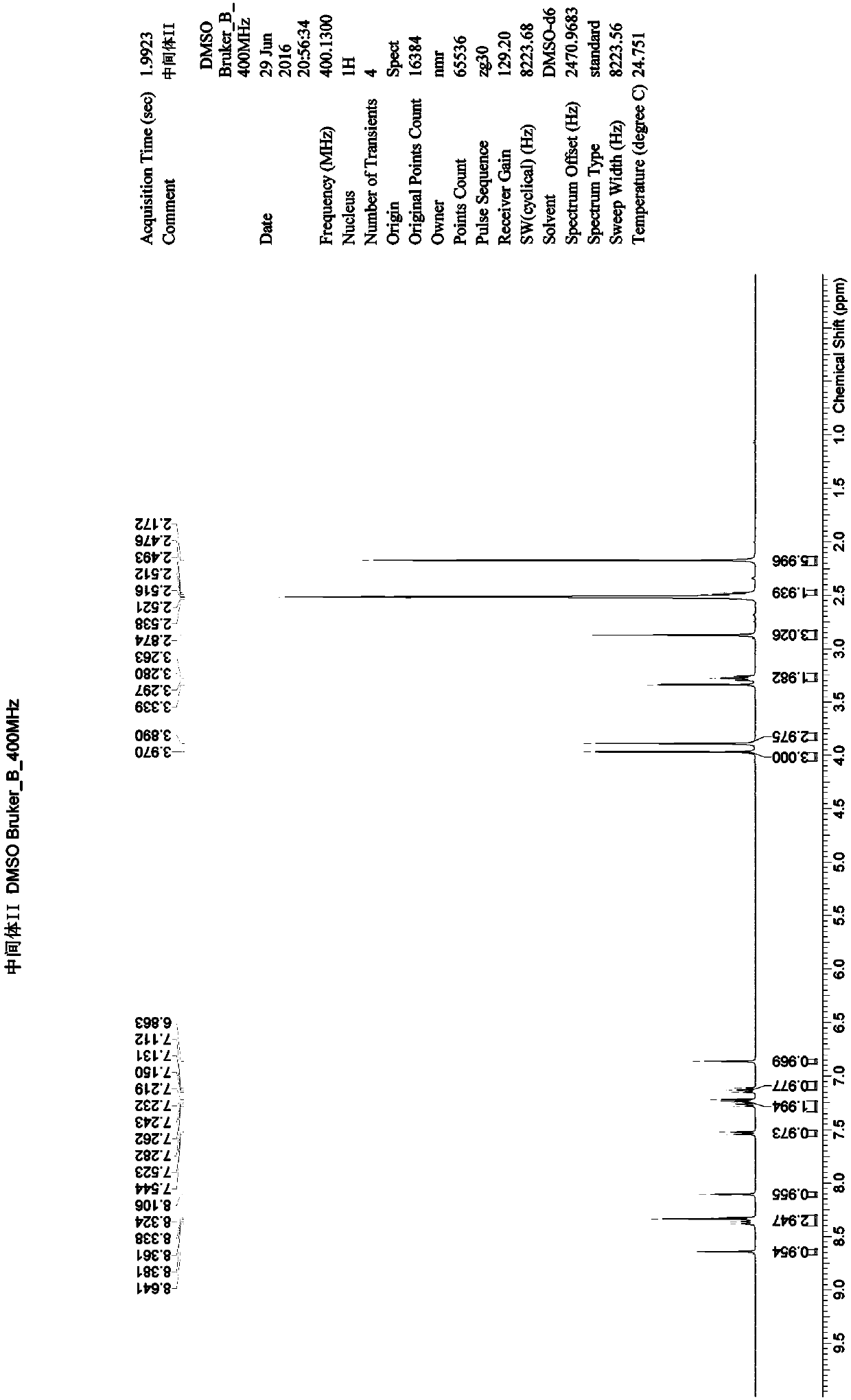
[0054] Example 2: N 4 -(2-Dimethylaminoethyl)-2-methoxy-N 4 -Methyl-N 1 Preparation of -[4-(1-methylindol-3-yl)-pyrimidin-2-yl]-5-nitro-benzene-1,4-diamine (MRTN-2)
[0055] Under nitrogen atmosphere, N-(4-fluoro-2-methoxy-5-nitro-phenyl)-4-(1-methylindole-3- base)-pyrimidin-2-amine (200.80g), N,N-dimethylacetamide (DMA, 1.95L), N,N',N'-trimethyl-ethylenediamine (SM3) (62.60g ), DIPEA (85.70g), after adding, heat up to 85°C, keep warm at 85°C-90°C, react for 5h-6h, TLC monitors the reaction, almost no intermediate I remains, the reaction is complete, cool down to room temperature, add water dropwise (975.00mL), after dropping, stir at 20°C to 30°C for 2h. After suction filtration, the filter cake was rinsed with ethanol (1.00 L) to obtain a light red wet material with a HPLC normalized purity of 98.44%. That is, get MRTN-2: N 4 -(2-Dimethylaminoethyl)-2-methoxy-N 4 -Methyl-N 1 -[4-(1-Methylindol-3-yl)-pyrimidin-2-yl]-5-nitro-benzene-1,4-diamine. 1 H-NMR (400Mz, DMSO-d...
Embodiment 3
[0056] Example 3: N 1 -(2-Dimethylaminoethyl)-5-methoxy-N 1 -Methyl-N 4 Preparation of -[4-(1-methylindol-3-yl)-pyrimidin-2-yl]-benzene-1,2,4-triamine (MRTN-3)
[0057] Put the wet material of MRTN-2 in Example 2 into the reaction flask, add ethanol (3.00L), replace the nitrogen, add stannous chloride (575.41g), and drop concentrated hydrochloric acid (163.16mL ), after dropping, stir for 10min. The temperature was raised to reflux, and the reaction was carried out at reflux for 3 hours. TLC monitored that there was no remaining raw material, and the temperature was lowered to room temperature. Suction filtration, rinse the filter cake with ethanol (500.00mL), and drain. While stirring, the filter cake was added to 5% aqueous sodium hydroxide solution, followed by dichloromethane (2.00 L), stirred for 10 min, and allowed to stand to separate layers. Collect the organic phase, filter the aqueous phase, extract the filtrate with 500.00 mL of dichloromethane, combine the org...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com