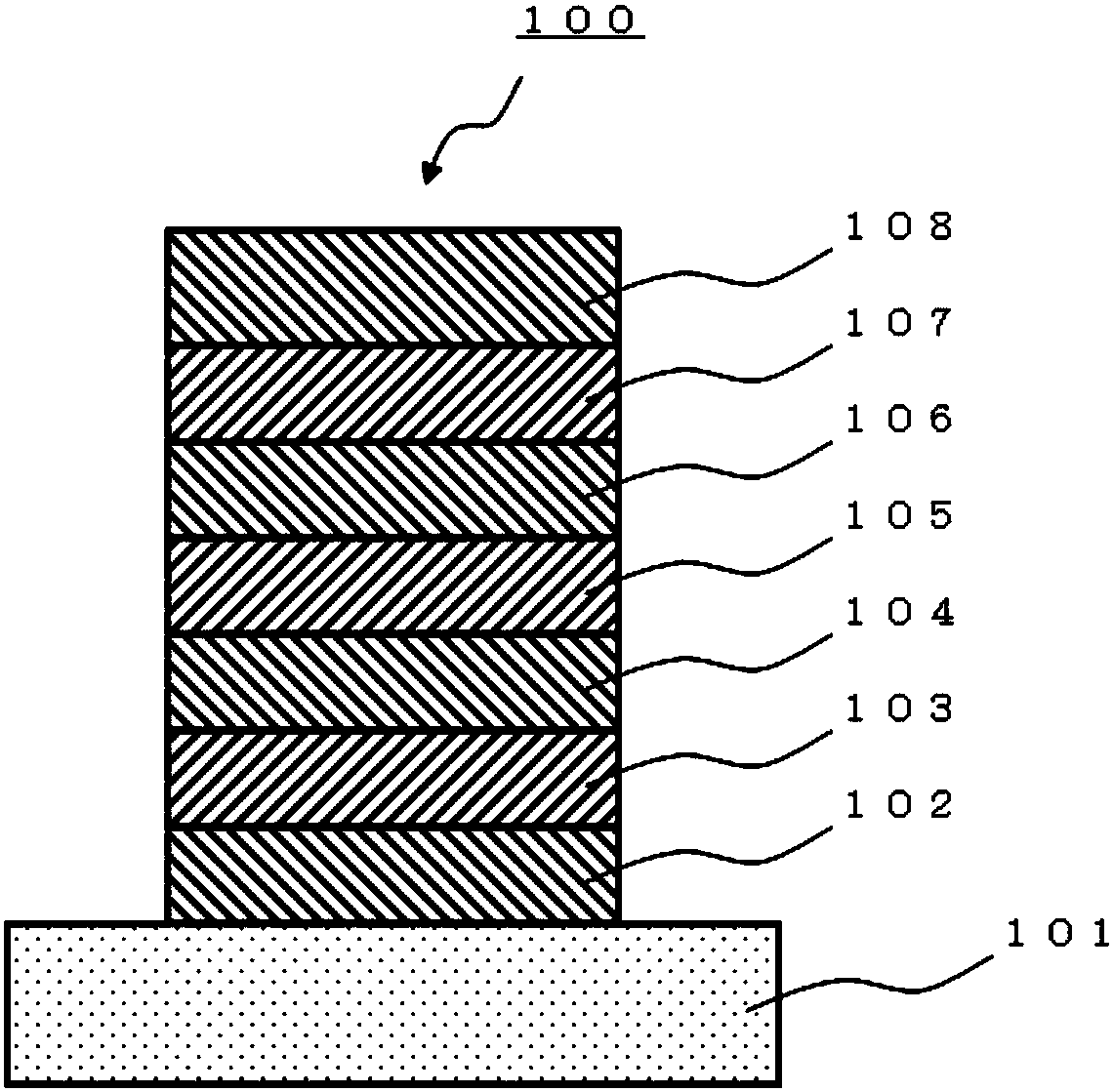
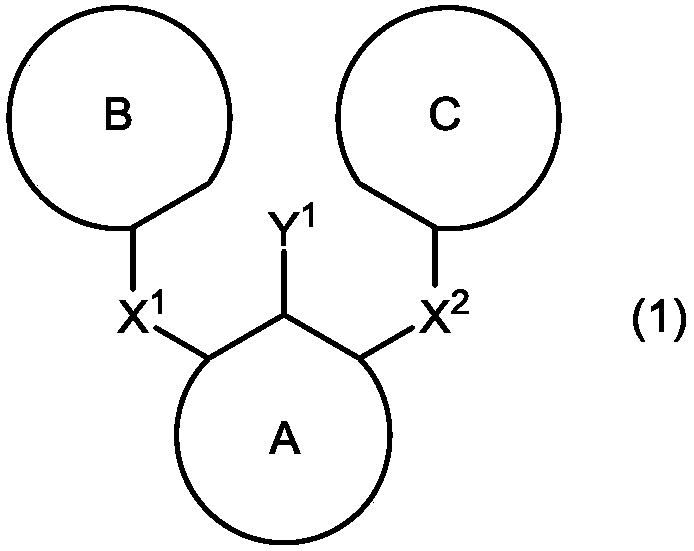
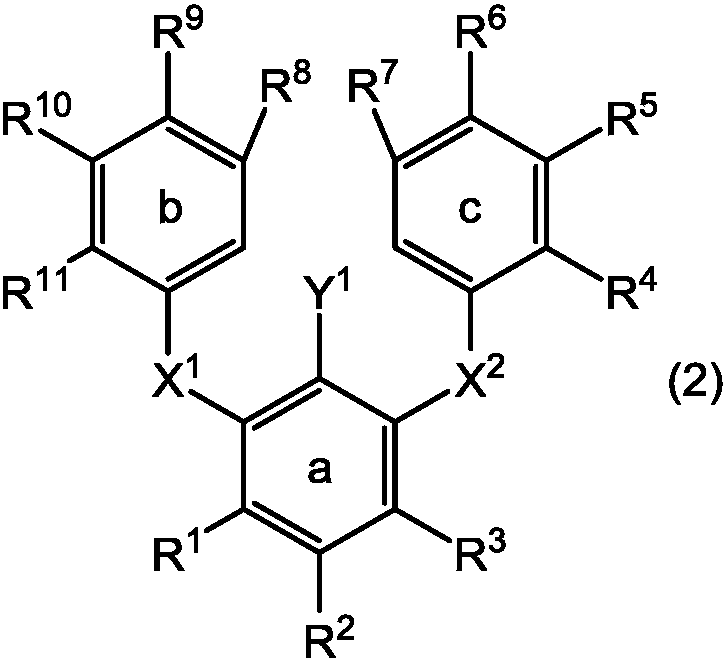
Boronic acid or boronic acid ester or method for producing polycyclic aromatic compound or oligomer of polycyclic aromatic compound by using same
A polycyclic aromatic compound technology, applied in the field of boronic acid and boronic acid ester, can solve the problems of lower yield, lower product selectivity, lower target product yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0262] Hereinafter, although an Example demonstrates this invention more concretely, this invention is not limited to them. First, a synthesis example of boric acid or boric acid ester will be described below.
Synthetic example (1
[0263] Synthesis Example (1): Synthesis of Compound (1-1-98)
[0264]
[0265] The flask containing Intermediate 1 (15 g) and toluene (300 ml) was heated to 70° C. under a nitrogen atmosphere to completely dissolve Intermediate 1 . After the flask was cooled to -20°C, tetramethylethylenediamine (20.8 g) and a 1.00 M mixed solution of sec-butyllithium in cyclohexane and n-hexane (89 ml) were added. After heating to 0°C and stirring for 3 hours, add 2-isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane (33.3g), and reflux for 1 hour . Water and toluene were added for liquid separation, and the solvent in the organic layer was distilled off under reduced pressure. The obtained solid was washed with Solmix A-11 (trade name: Japan Alcohol Trading Co., Ltd.), dissolved in toluene, and left standing at 0° C. for 1 hour. The deposited precipitate was separated by filtration, and the filtrate was passed through a silica gel column (eluent: toluene / heptane=1 / 5 mixed solvent (volume...
Synthetic example (2
[0269] Synthesis Example (2): Synthesis of Compound (1-3-139)
[0270]
[0271] A flask charged with 1-bromo-2,6-difluorobenzene (85.4g), phenol (100g), potassium carbonate (244.7g) and N-methyl-2-pyrrolidone (NMP, 300ml) was placed under a nitrogen atmosphere The temperature was raised to 180° C. and stirred for 22 hours. After the reaction was completed, potassium carbonate was separated by filtration at 150° C., and the solvent of the filtrate was distilled off under reduced pressure. The obtained solid was dissolved in toluene and passed through a silica gel short-pass column (eluent: toluene). After the solvent was distilled off under reduced pressure, the obtained solid was washed with heptane to obtain Intermediate 2 (126.6 g) as a white solid.
[0272]
[0273] To the flask charged with Intermediate 2 (103 g) and tetrahydrofuran (500 ml) was added a 1.29 M tetrahydrofuran solution (351 ml) of isopropylmagnesium chloride·lithium chloride complex at room temperat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com