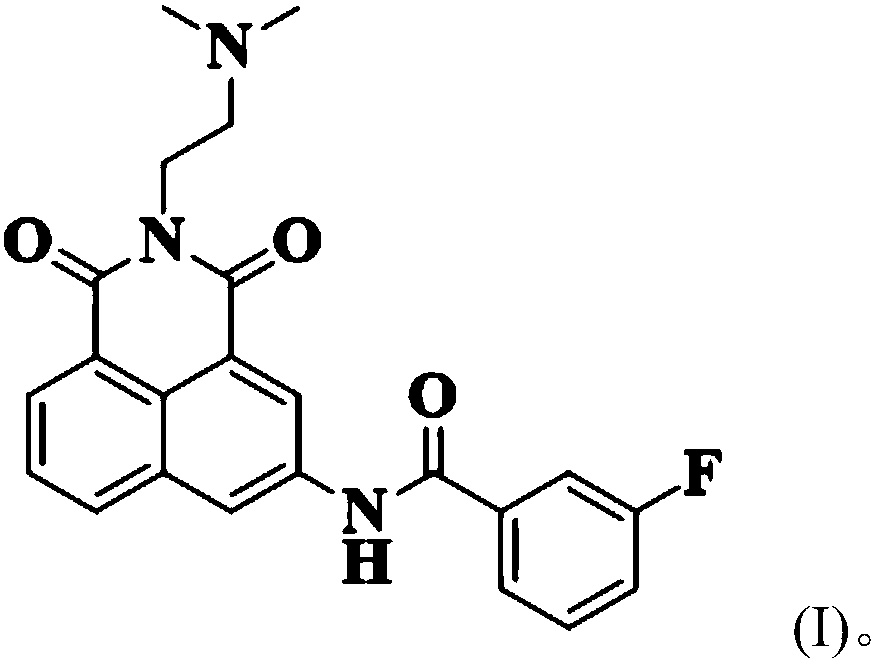
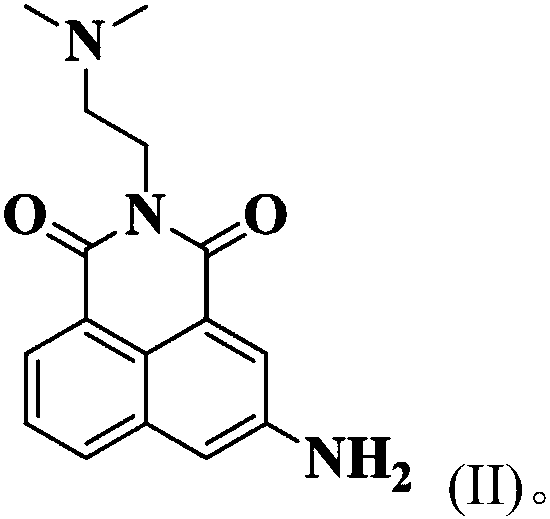
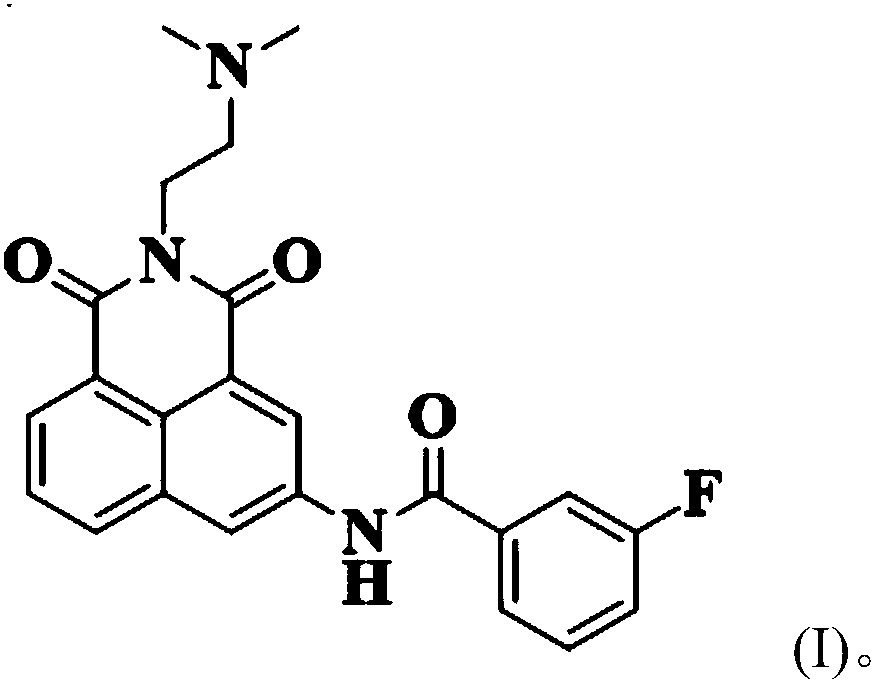
Low-toxicity 1,8-naphthalene dimethimide derivative as well as preparation method and application thereof
A synthesis method and reaction technology, applied in the field of medicine, can solve the problems of retention, adverse reactions, toxic and side effects, etc., and achieve the effects of small toxic and side effects, significant inhibitory activity, and significant biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Get 1mmol of naphthalene, add it to 4mL of acetonitrile and stir to dissolve, dissolve 1.5mmol of 3-fluorobenzoyl chloride in 2mL of acetonitrile, put it in a constant pressure dropping funnel, and use nitrogen to protect the reaction system, and pour into the nadafetil-acetonitrile solution Slowly add 3-fluorobenzoyl chloride-acetonitrile solution dropwise, react for 2.5h after the dropwise addition is completed, remove the solvent after the reaction, and purify using silica gel column chromatography (dichloromethane:methanol=20:1, v / v) , to obtain a pale yellow solid (about 90% yield).
[0032] The obtained light yellow solid is identified:
[0033] (1) Proton nuclear magnetic resonance spectrum and carbon spectrum, their spectrogram data are as follows:
[0034] 1 H NMR (500MHz, DMSO-d6) δ11.05(s, 1H), 9.00(d, J=1.9Hz, 1H), 8.93(d, J=2.0Hz, 1H), 8.42(dd, J=12.0, 7.7Hz, 2H), 8.02–7.91(m, 2H), 7.85(t, J=7.7Hz, 1H), 7.65(dd, J=13.9, 7.9Hz, 1H), 7.51(td, J=8.5, 2.1 H...
Embodiment 2
[0040] Repeat Example 1, the difference is:
[0041] The reaction time was changed to 1 h, and the rest of the reaction conditions remained unchanged (the yield was about 73%).
[0042] The product obtained in this example was analyzed by proton nuclear magnetic resonance spectrum and carbon nuclear magnetic resonance spectrum, and it was determined to be the target product.
Embodiment 3
[0044] Repeat Example 1, the difference is:
[0045] The reaction time was changed to 3 h, and the rest of the reaction conditions remained unchanged (the yield was about 88%).
[0046] The product obtained in this example was analyzed by proton nuclear magnetic resonance spectrum and carbon nuclear magnetic resonance spectrum, and it was determined to be the target product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com