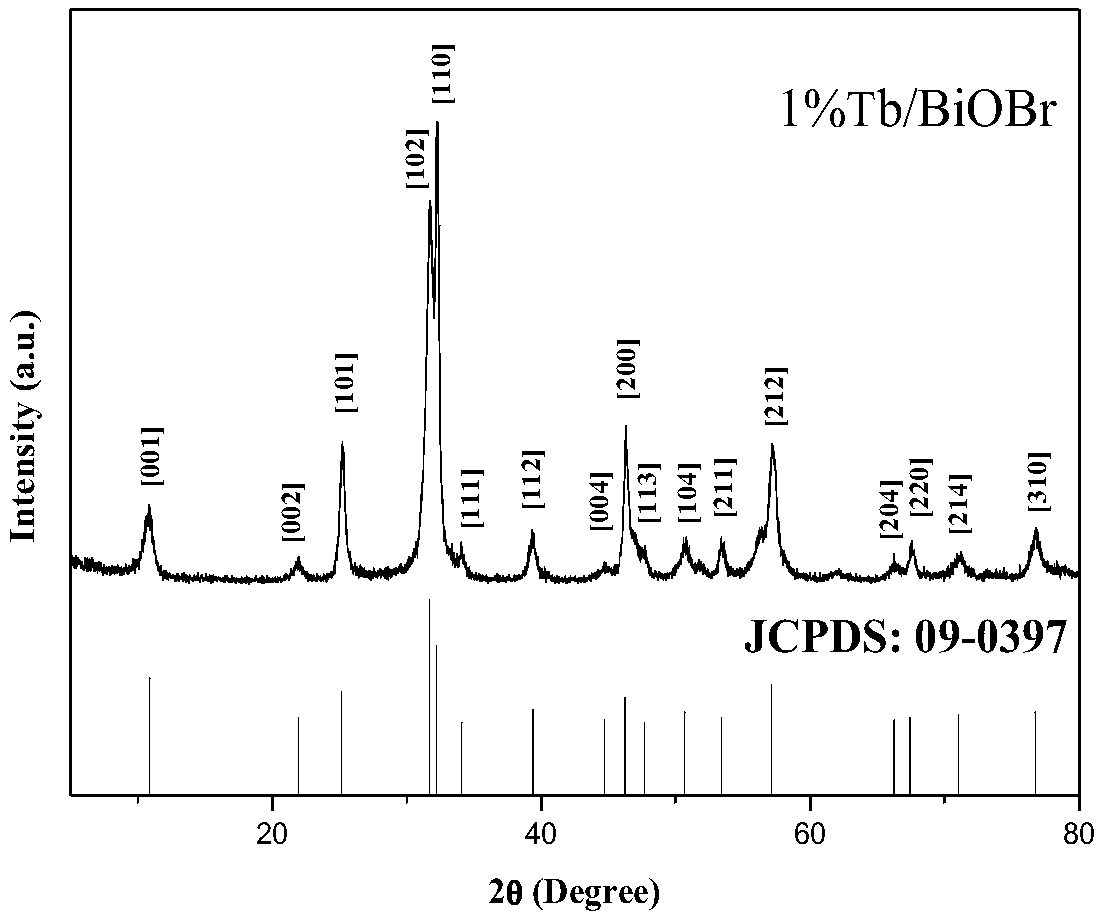
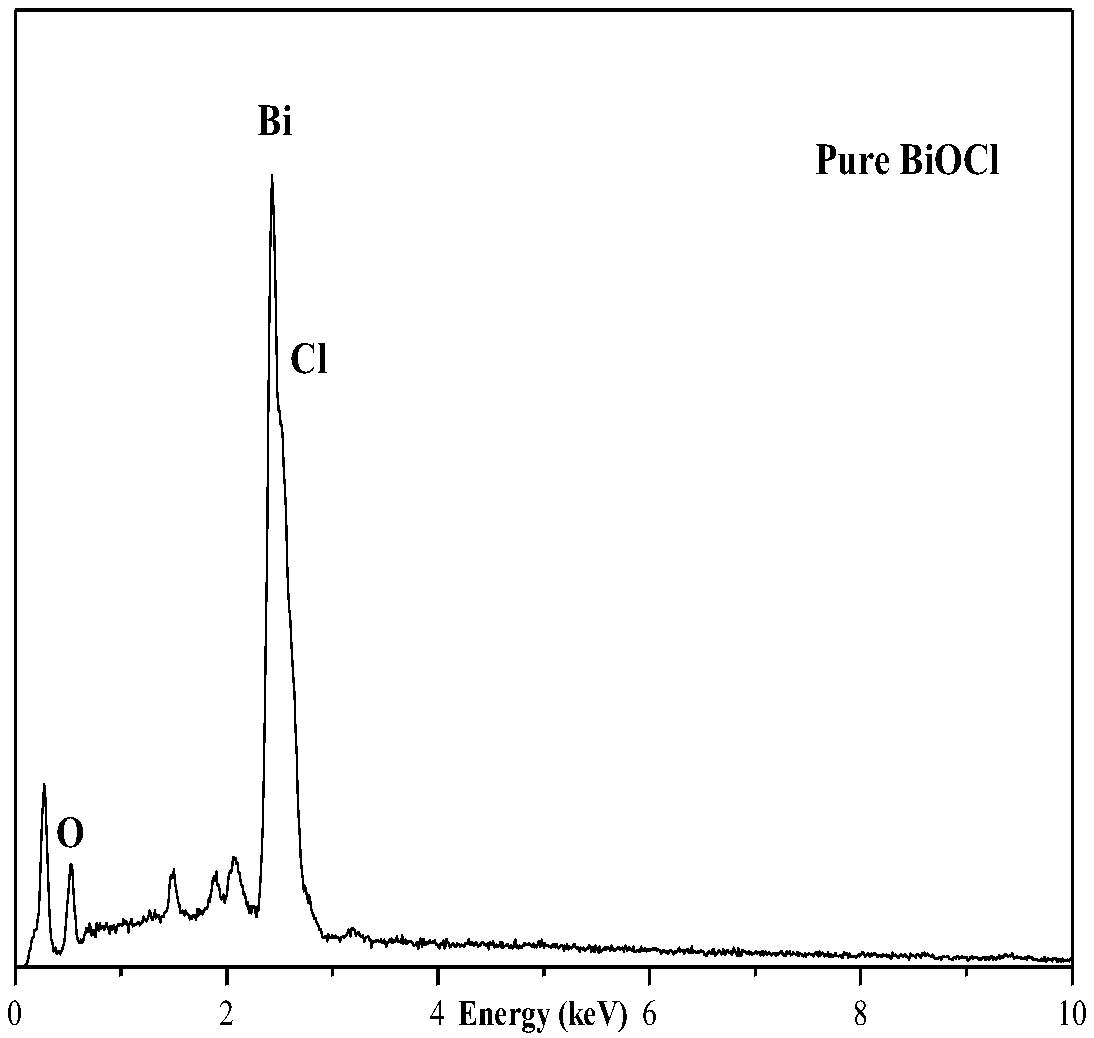
Method for preparing composite nano Tb/BiOCl material
A composite, nano-technology, applied in the field of photocatalytic materials, achieves the effects of simple preparation method, reduced probability and high catalytic degradation ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] 1) At room temperature, take 17mL (CH 2 Oh) 2 In a beaker, add 0.2g of KCl until a clear and transparent solution A is formed; take 17mL (CH 2 Oh) 2 In the three-necked flask, add 0.9g of Bi(NO 3 ) 3 ·5H 2 O, mechanical stirring until forming a clear and transparent solution B;
[0039] 2) Add solution A dropwise to solution B, continue to stir vigorously for 30 minutes after the dropwise addition, add Tb(NO 3 ) 3 ·6H 2 O, and Tb(NO 3 ) 3 ·6H 2 The amount of O added is Bi(NO 3 ) 3 ·5H 2 1% of the molar amount of O, placed in an ultrasonic cleaner and oscillated at 40kHz for 15min;
[0040] 3) Stop the ultrasonic vibration, transfer the obtained solution into a 40mL stainless steel Teflon-lined reaction kettle, seal it and put it in an oven at 180°C for 9 hours;
[0041] 4) The reaction kettle was naturally cooled to room temperature, the liquid in the reaction kettle was poured out, the solid precipitate was collected, washed with deionized water and etha...
Embodiment 2
[0043] 1) At room temperature, take 17mL (CH 2 Oh) 2 In a beaker, add 0.28g of KCl until a clear and transparent solution A is formed; take 17mL (CH 2 Oh) 2 In the three-necked flask, add 0.97g of Bi(NO 3 ) 3 ·5H 2 O, mechanical stirring until forming a clear and transparent solution B;
[0044] 2) Add solution A dropwise to solution B, continue to stir vigorously for 30 minutes after the dropwise addition, add Tb(NO 3 ) 3 ·6H 2 O, and Tb(NO 3 ) 3 ·6H 2 The amount of O added is Bi(NO 3 ) 3 ·5H 2 1% of the molar amount of O, placed in an ultrasonic cleaner and oscillated at 40kHz for 15min;
[0045] 3) Stop the ultrasonic vibration, transfer the obtained solution into a 40mL stainless steel Teflon-lined reaction kettle, seal it and put it in an oven at 180°C for 9 hours;
[0046] 4) The reaction kettle was naturally cooled to room temperature, the liquid in the reaction kettle was poured out, the solid precipitate was collected, washed with deionized water and et...
Embodiment 3
[0048] 1) At room temperature, take 17mL (CH 2 Oh) 2 In a beaker, add 0.3g of KCl until a clear and transparent solution A is formed; take 17mL (CH 2 Oh) 2 In the three-necked flask, add 1g of Bi(NO 3 ) 3 ·5H 2 O, mechanical stirring until forming a clear and transparent solution B;
[0049] 2) Add solution A dropwise to solution B, continue to stir vigorously for 30 minutes after the dropwise addition, add Tb(NO 3 ) 3 ·6H 2 O, and Tb(NO 3 ) 3 ·6H 2 The amount of O added is Bi(NO 3 ) 3 ·5H 2 1% of the molar amount of O, placed in an ultrasonic cleaner and oscillated at 40kHz for 15min;
[0050] 3) Stop the ultrasonic vibration, transfer the obtained solution into a 40mL stainless steel Teflon-lined reaction kettle, seal it and put it in an oven at 180°C for 9 hours;
[0051] 4) The reaction kettle was naturally cooled to room temperature, the liquid in the reaction kettle was poured out, the solid precipitate was collected, washed with deionized water and ethano...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com