Preparation method of venetoclax key intermediate
An intermediate and key technology, applied in the field of pharmaceutical synthesis, can solve the problems of many reaction impurities, low synthesis process yield, high price, etc., and achieve the effects of optimizing the preparation process, shortening the process flow, and reducing the production cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
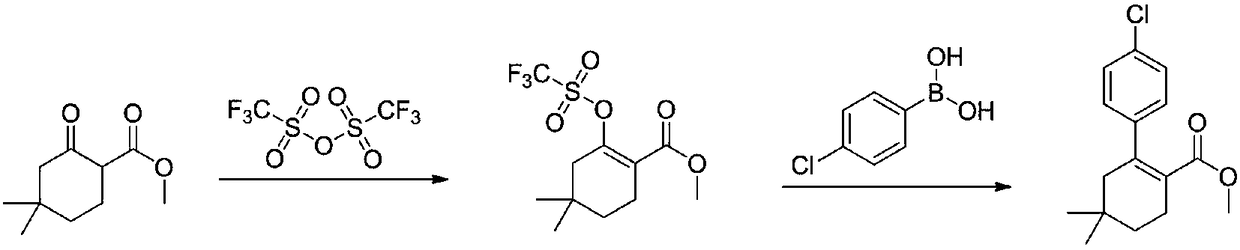
[0033] The first step: the synthesis of methyl 4,4-dimethyl-2-cyclohexanone carboxylate
[0034] In a reaction flask, dissolve 100 g (0.79 mol) of 3,3-dimethylcyclohexanone in 500 mL of tetrahydrofuran, cool to -20°C to -30°C, and add dropwise 1mol / L LDA (lithium diisopropylamide ) tetrahydrofuran solution 870mL (0.87mol), warm up to 0°C, stir for 1 hour, cool down to -20°C, add 93g (1.03mol) of dimethyl carbonate dropwise, rise to 0°C, react for 2 hours, TLC monitors that the reaction is complete , add 1M dilute hydrochloric acid to adjust the pH to 6-7, separate the layers, extract the aqueous phase with ethyl acetate twice, combine the organic phases, dry over sodium sulfate, and concentrate under reduced pressure to obtain 4,4-dimethyl-2-cyclohexanone 134.3 g of methyl formate, yield 92%.
[0035] HNMR(400MHz, CDCl3):3.74(s,3H),3.73(m,3H),2.24(m,2H),2.04(s,2H),
[0036] 1.37(t,J=6.4Hz,2H),0.94(s,6H).
[0037] The second step: the synthesis of methyl 2-(4-chlorophenyl)-4...
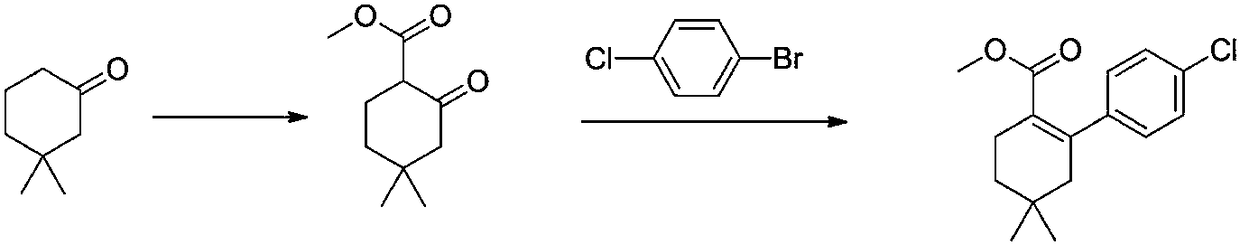
example example 2
[0041] The first step: with reference to the operation of the first step in Example 1, change the reaction conditions to obtain the results in Table 1.
[0042] Table 1
[0043] experiment number
Alkali class
temperature reflex
yield
1
LDA
-20°C to 0°C
85%
2
LDA
-70°C to -60°C
92%
3
HMDSL
-30°C to -20°C
90%
4
t-BuOK
-20°C to 0°C
0%
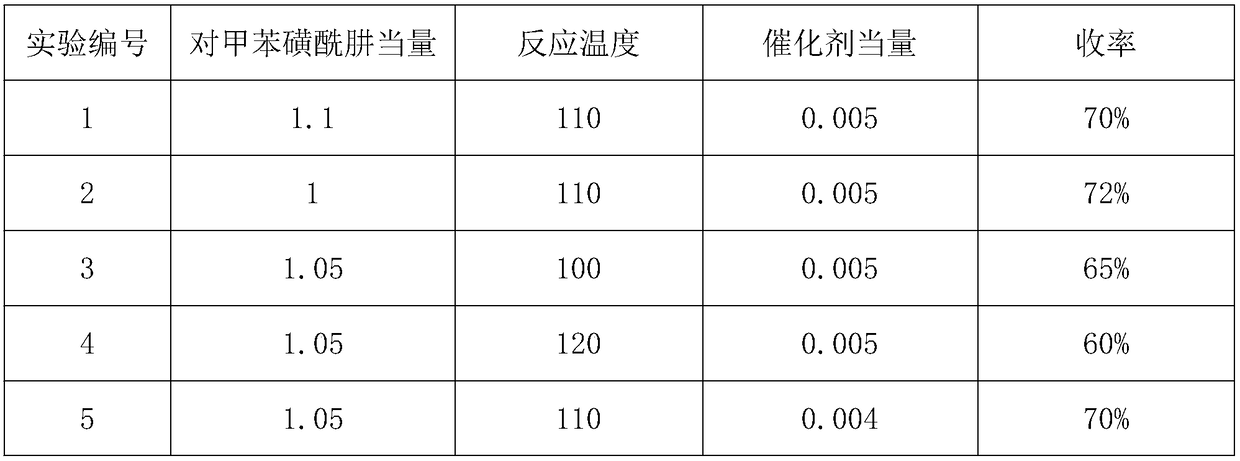
[0044] The second step: with reference to the second step operation of the embodiment, change the following conditions to obtain the results in Table 2
[0045] Table 2
[0046]
[0047]
[0048] Experiment number 7* is to replace p-toluenesulfonylhydrazide with 2,4,6-triisopropylbenzenesulfonylhydrazide.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com