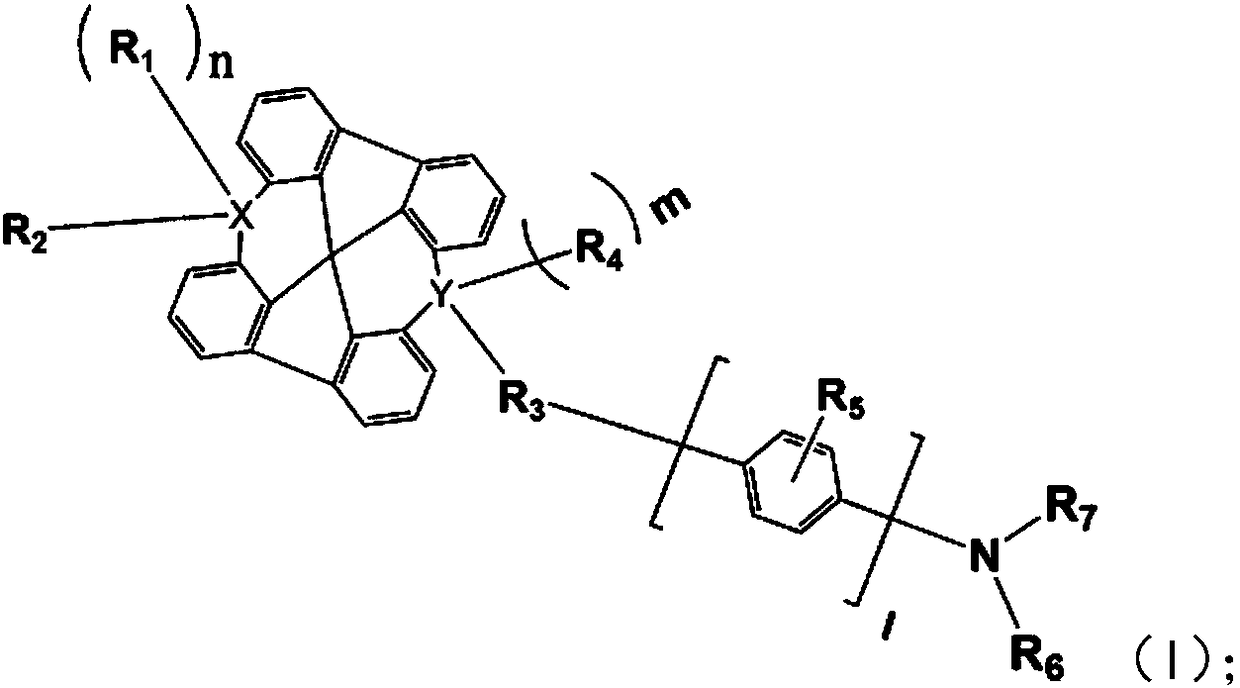
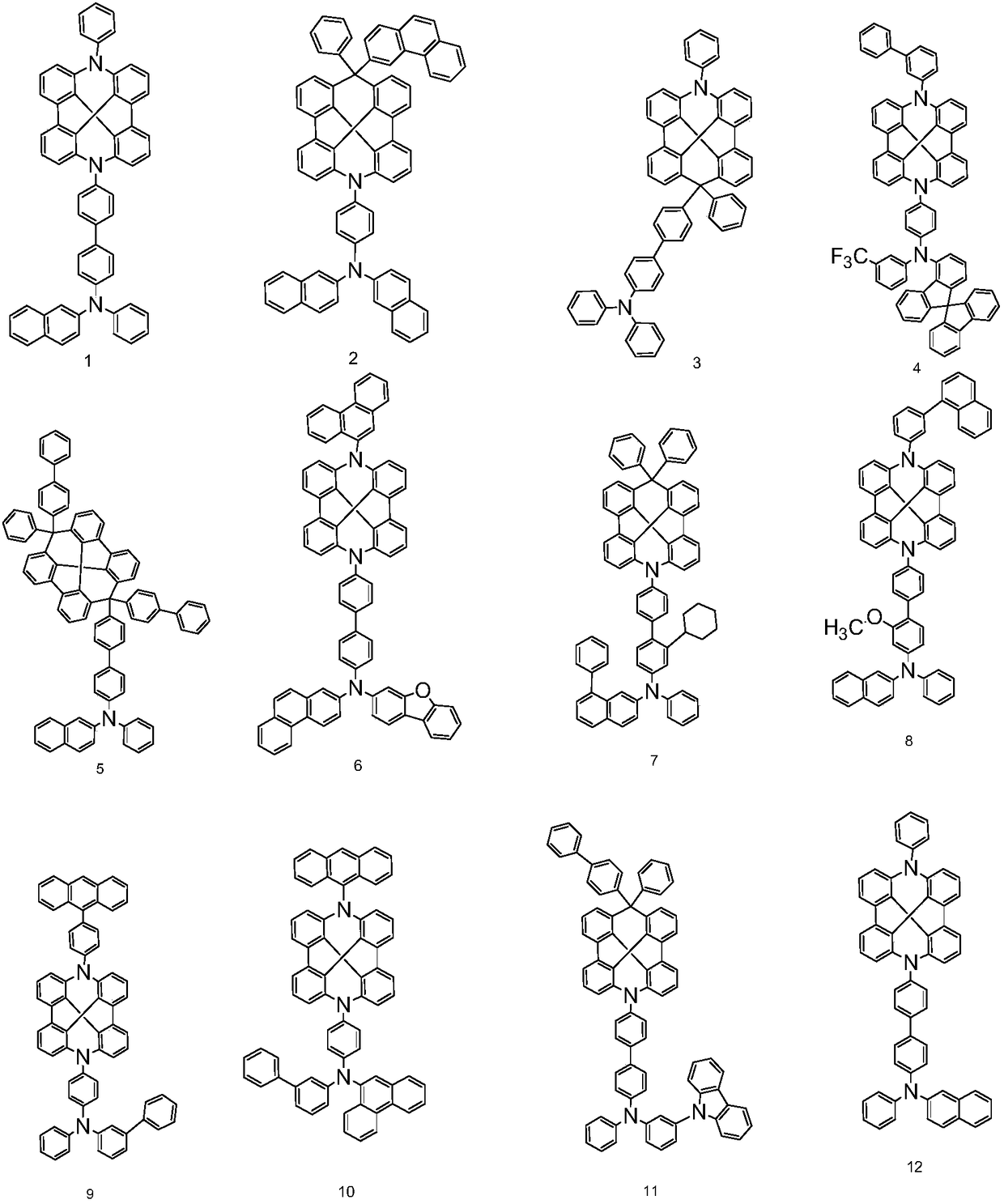
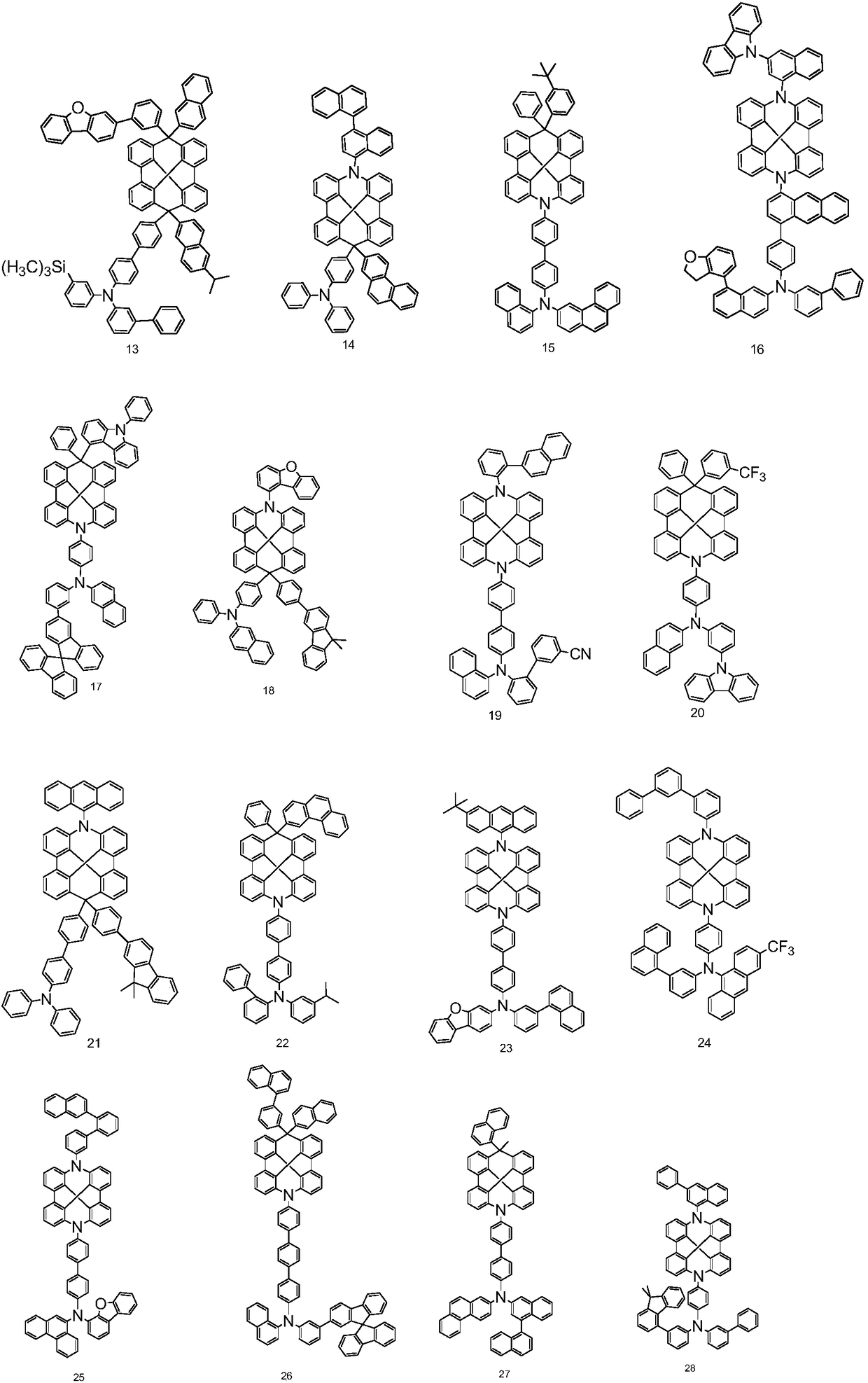
Novel organic light-emitting compound, application thereof and organic light-emitting device adopting compound
An electroluminescence and compound technology, applied in the field of organic electroluminescence devices, can solve the problems of high driving voltage, low luminous efficiency, low brightness, thermal stability, color purity and device life, and achieve low driving voltage, excellent current and power efficiency. , Improve the effect of driving life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] The synthesis of embodiment 1 compound 1
[0064] Synthesis of Intermediate-1
[0065] [Reaction 1]
[0066]
[0067] In a dry 3L three-necked flask, add 21.9g (100mmol, 1.0eq.) of N-phenyl-2-naphthylamine and 43.1g (120mmol, 1.2eq.) of 4-bromo-4-iodobiphenyl, then add dry And degassed 1300ml of toluene was used as a solvent, and nitrogen gas was passed for 15 minutes. Then 2.9 g (15% mol) of cuprous iodide, 5.9 g (30% mol) of 1,10-phenanthroline and 63.7 g (300 mol, 3 eq.) of potassium phosphate were added. The temperature was raised to 110°C, and the reaction was carried out for 18 hours. After the reaction was completed, it was cooled to room temperature, suction filtered, the solvent was spun off, and recrystallized with toluene and ethanol to obtain 37.4 g of Intermediate-1 with a yield of 83%.
[0068] Synthesis of Intermediate-2
[0069] [Reaction 2]
[0070]
[0071] In dry 3L there-necked flask, add 16.2g (83mmol, 1.0eq.) Acridone and 37.4g (83mmol,...
Embodiment 2
[0091] The synthesis of embodiment 2 compound 37
[0092] Synthesis of Intermediate-6
[0093] [Reaction 6]
[0094]
[0095] In a dry 3L three-necked flask, add 18.4g (120mmol, 1.2eq.) 4-aminobiphenyl and 24.7g 2-bromodibenzofuran (100mmol, 1.0eq.), then add dry and degassed 900ml toluene As a solvent, nitrogen gas was passed for 15 minutes. Then add 19.2g (200mmol, 2.0eq.) sodium tert-butoxide, 1.8g (2%mol) catalyst Pd2(dba)3 and 8.1ml (4%mol) P(t-bu) 3 Toluene solution (m / v, 10%). The temperature was raised to 100°C, and the reaction was carried out for 6 hours. After the reaction was completed, cool to room temperature, add activated carbon for adsorption, filter with suction, spin off the solvent, and recrystallize with toluene and ethanol to obtain 27.2 g of Intermediate-6 with a yield of 81%.
[0096] Synthesis of Intermediate-7
[0097] [Reaction 7]
[0098]
[0099] Add 18g (81mmol, 1.0eq.) p-iodofluorobenzene and 37.4g (81mmol, 1.0eq.) Intermediate-6 in ...
Embodiment 3
[0127] The synthesis of embodiment 3 compound 54
[0128] Synthesis of Intermediate-13
[0129] [Equation 14]
[0130]
[0131] Add 33g (100mmol, 1.0eq.) m-diiodobenzene and 20.3g (120mmol, 1.2eq.) 3-aminobiphenyl in a dry 3L three-necked flask, then add dry and degassed 1000ml toluene as a solvent, pass Nitrogen for 15 minutes. Then add 19.2g (200mmol, 2.0eq.) sodium tert-butoxide, 1.8g (2%mol) catalyst Pd 2(dba) 3 and 8.1ml (4%mol) P(t-bu) 3 Toluene solution (m / v, 10%). The temperature was raised to 100°C, and the reaction was carried out for 2 hours. After the reaction was cooled to room temperature, activated carbon was added for adsorption, suction filtered, the solvent was spun off, and recrystallized with toluene and ethanol to obtain 31.9 g of Intermediate-13 with a yield of 86%.
[0132] Synthesis of Intermediate-14
[0133] [Equation 15]
[0134]
[0135] Add 31.9g (86mmol, 1.0eq.) of Intermediate-13 and 25.9g (86mmol, 1.0eq.) of 2-bromo-1-fluoro-3-iod...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com