A kind of low-temperature matrix metalloproteinase and its coding gene and application
A technology of matrix metals and coding genes, applied in the field of enzyme engineering, can solve the problems of lack of oxygen supply, damage to cell culture activity, etc., and achieve the effect of high-efficiency protein degradation activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
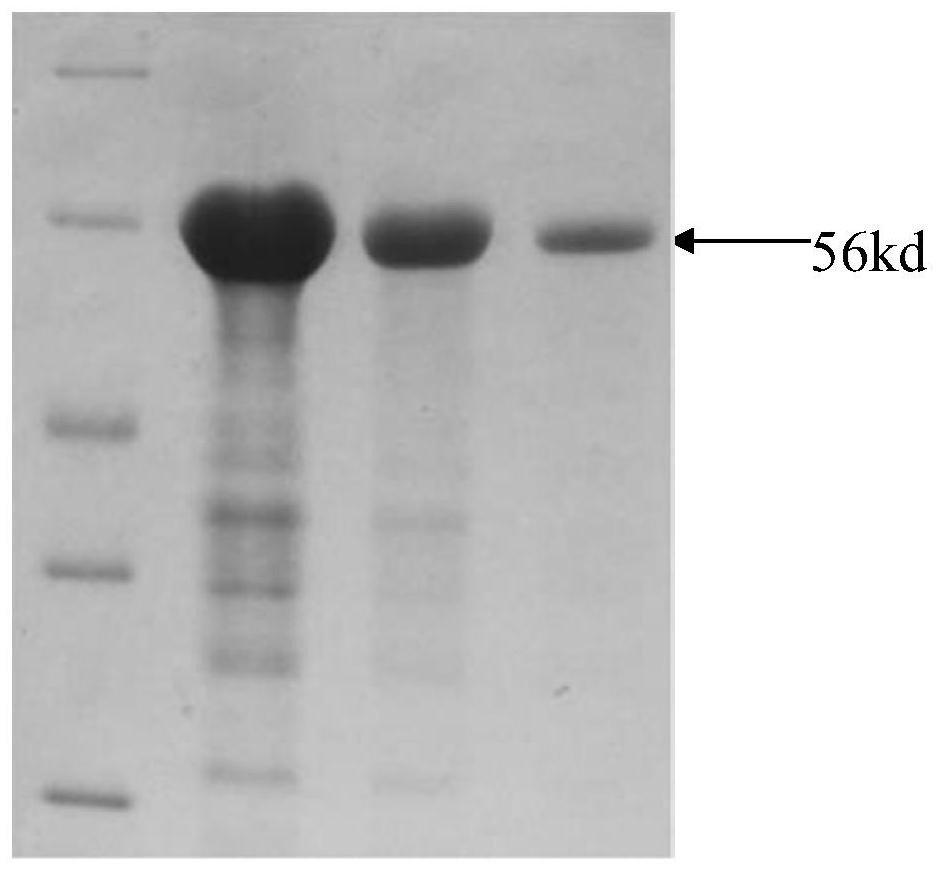
[0035] Example 1 Obtaining of low-temperature matrix metalloproteinase gene and expression of low-temperature matrix metalloproteinase 1. Establishment of low-temperature matrix metalloproteinase gene transcription library
[0036] The adult Antarctic fish of Roche (Notothenia rossii) was dissected by vivisection, and the mouth samples were taken, which were quick-frozen in liquid nitrogen and stored at -80°C for later use. After tissue samples were ground with liquid nitrogen, total RNA was extracted with RNAiso Plus (Takara, Dalian) kit. RNA samples were digested with RNase-free DNase I to remove genomic DNA contamination, then detected by agarose gel electrophoresis and UV spectrophotometer, and the concentration was adjusted to 500ng / μL. Using the above RNA sample as a template, it was reverse-transcribed into cDNA by AMV reverse transcription kit (Promega), and stored at -20°C.
[0037] 2. Acquisition of low temperature matrix metalloproteinase gene
[0038] Using the c...
Embodiment 2
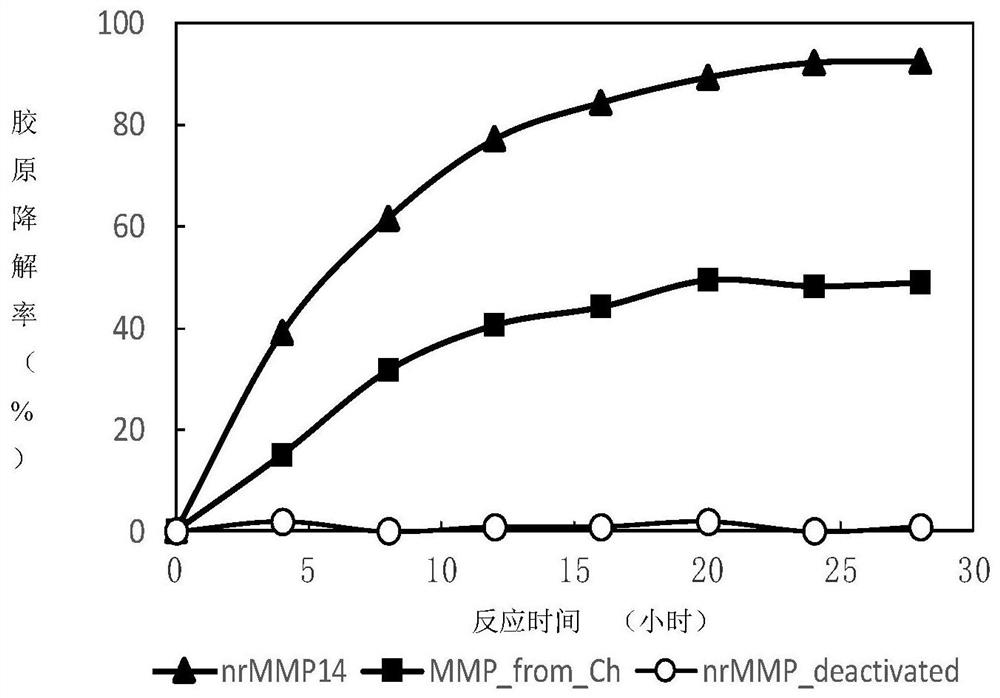
[0047] Example 2 Activity Detection of Low Temperature Matrix Metalloproteinases (Hydroxyproline Detection Method)
[0048] 2.1 Test principle
[0049] Matrix metalloproteinase catalyzes the decomposition of solid insoluble collagen into soluble peptides and enters the solution. After the reaction is terminated, unreacted proteins are filtered out, and the filtrate is heated and acidified to free amino acids. Hydroxyproline is a specific amino acid of collagen, and the quality of free hydroxyproline in the acid hydrolysis solution is measured by the hydroxyproline colorimetric method, which indirectly indicates the amount of hydrolyzed collagen. The ratio of this value to the total content of hydroxyproline in the substrate collagen is called the degradation rate of collagen, which can reflect the collagen hydrolysis activity of matrix metalloproteinases.
[0050] 2.2 Experimental method
[0051] With Tris-HCl buffer (pH=7.4, containing 5mmol / ml CaCl 2 , 0.5mmol acetic acid...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com