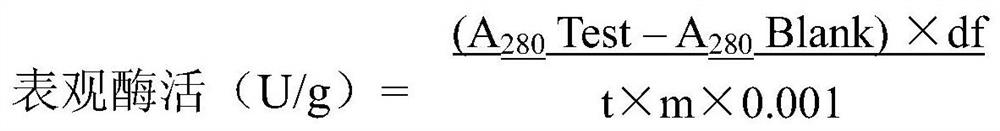A kind of low-temperature acid protease and its coding gene and application
A technology of acid protease and encoding gene, which is applied to low-temperature acid protease and its encoding gene and application field to achieve the effect of high-efficiency protein degradation activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
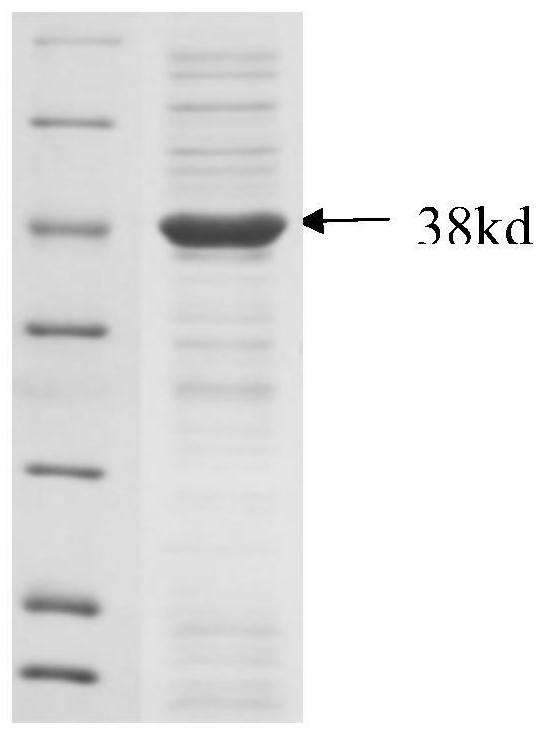
[0034] The acquisition of embodiment 1 low-temperature acid protease gene and its expression
[0035] 1. Establishment of low temperature acid protease gene transcription library
[0036] The adult Antarctic fish (Notothenia rossii) was vivisectioned, and the gastric mucosa samples were taken, quickly frozen in liquid nitrogen, and stored at -80°C for later use. After the tissue samples were ground with liquid nitrogen, total RNA was extracted with the RNAiso Plus (Takara, Dalian) kit. RNA samples were digested with RNase-free DNase I to remove genomic DNA contamination, then detected by agarose gel electrophoresis and UV spectrophotometer, and the concentration was adjusted to 500 ng / μL. Using the above RNA sample as a template, it was reverse-transcribed into cDNA by AMV Reverse Transcription Kit (Promega), and stored at -20°C.
[0037] 2. Acquisition of low temperature acid protease gene
[0038] With the cDNA obtained in step 1 as template, utilize primer 1 shown in Seq...
Embodiment 2
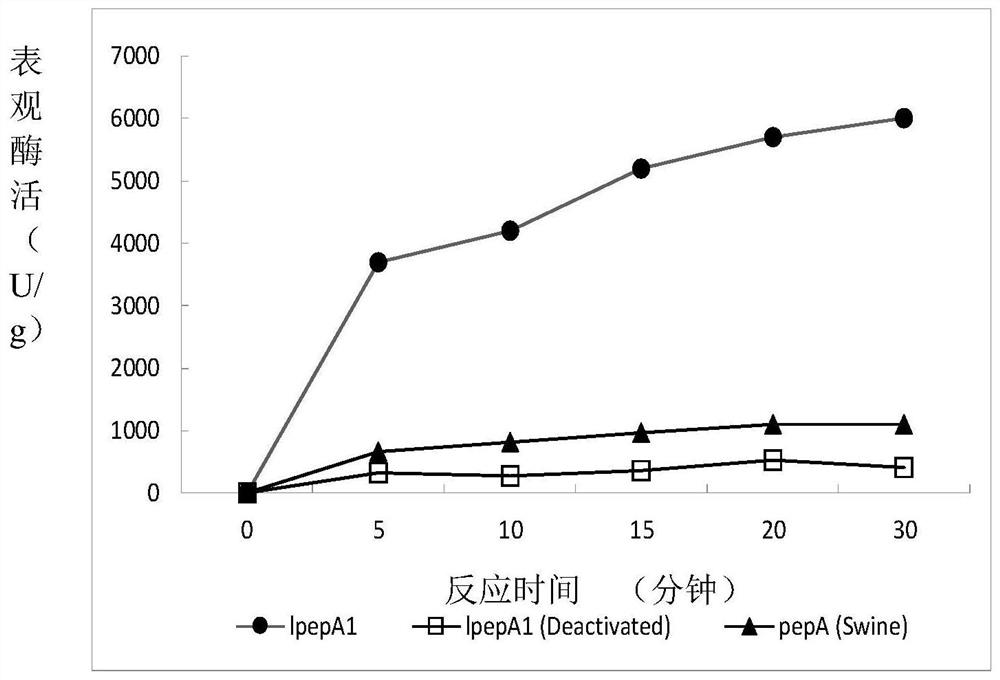
[0046] The activity detection of embodiment 2 low-temperature acid protease (hemoglobin hydrolysis method)
[0047] 1. Principle: The target substrate, bovine hemoglobin, is hydrolyzed by acid protease under appropriate buffer conditions to release free tyrosine or small peptides containing tyrosine into the reaction solution. Afterwards, trichloroacetic acid was added to the reaction system to precipitate macromolecular proteins (including acid protease and unreacted substrate hemoglobin), and the content of tyrosine in the supernatant was determined by colorimetry, which could indirectly reflect the activity level of acid protease.
[0048] 2. Experimental methods and results:
[0049] 2.1 Test sample
[0050] Low temperature acid protease lpepA1 (derived from Example 1); commercially available porcine pepsin (pepA (Swine)).
[0051] In the test of the above samples, the reaction test tubes of "experimental group" and "blank control group" were respectively set up to measu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com