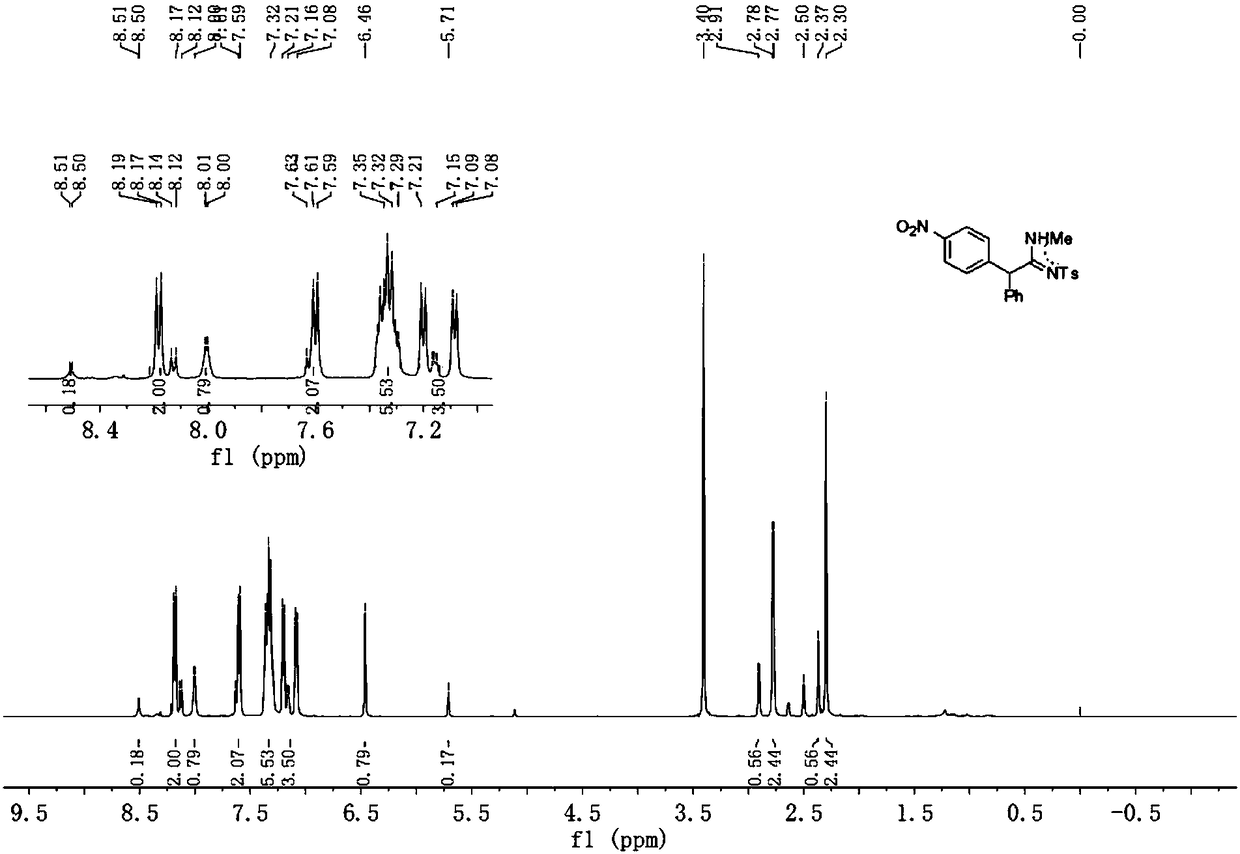
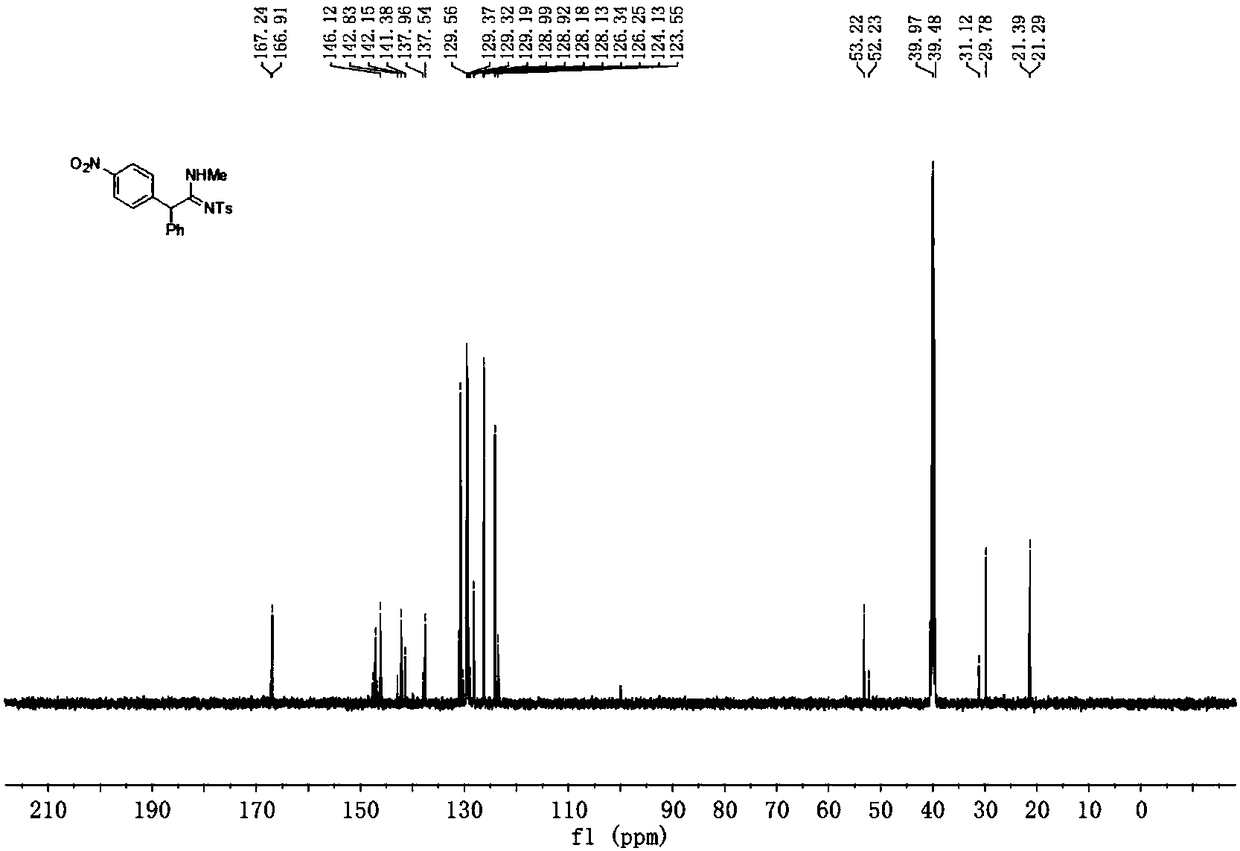
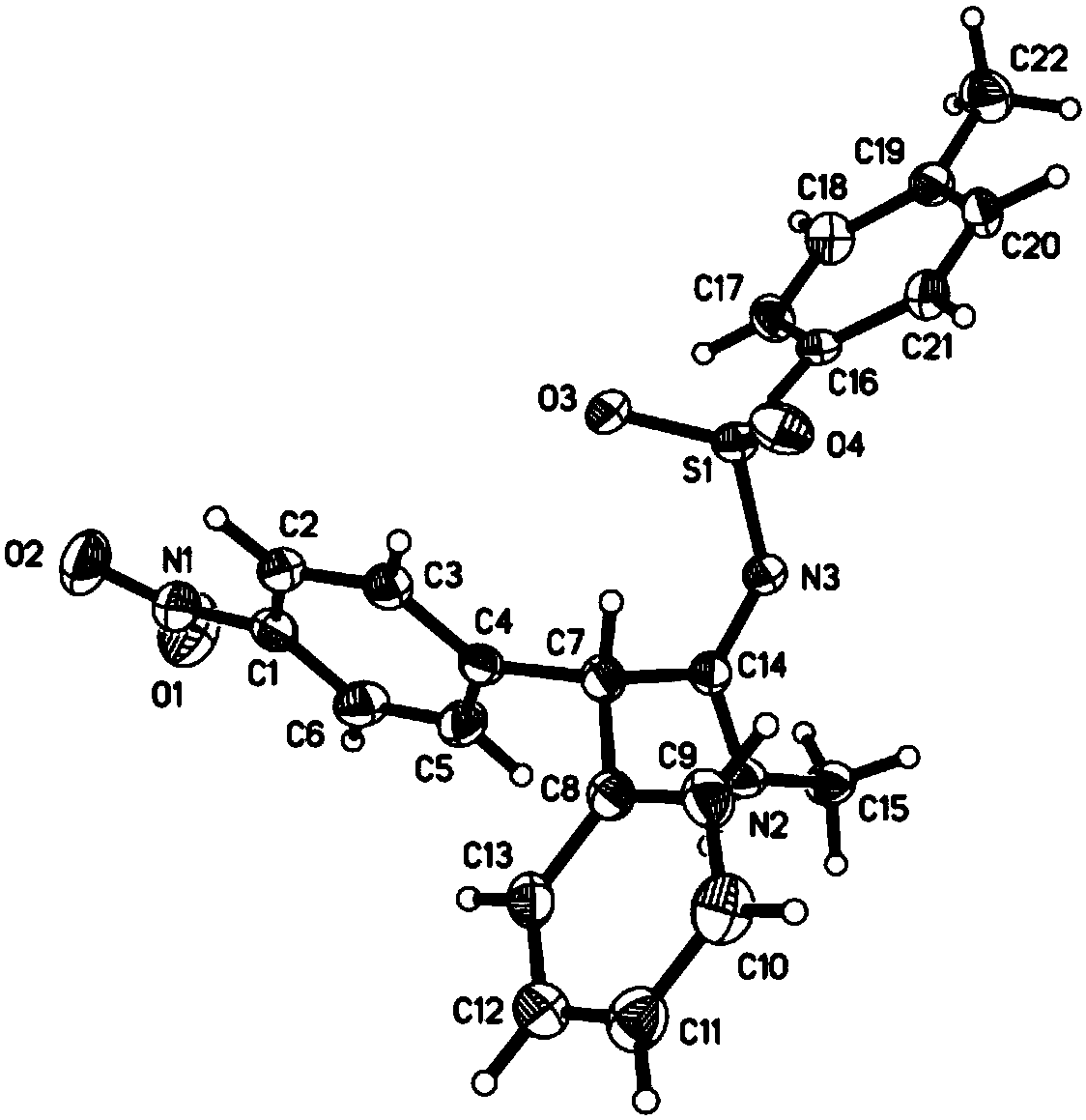
Preparation method of alpha-arylamidine imine derivative
A technology for aryl amidine imines and derivatives, which is applied in the field of preparation of α aryl amidine imine derivatives, can solve problems such as low yield and complex process, achieve high yield, improve reaction efficiency, and facilitate industrial application foreground effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] A kind of preparation method of α aryl amidine imine derivative, its preparation process specifically comprises the following steps:
[0034] (1), add the cuprous catalyst of magnetic stirring bar and 0.075mmol (10.5mg) and the N-R1 of 0.5mmol (146mg) in 10ml sealing tube, the benzene sulfonamide that R2 replaces, nitrogen replacement 3-5 times, then Inject 1.5mmol (152mg) of R3-substituted terminal alkynes and 1.5mmol (296mg) of R4-substituted sulfonyl azide with a 100ul microsyringe, and inject 2ml of organic solvent dichloromethane into it with a 2.5ml syringe under nitrogen protection , and then add 1mmol (140μL) additive dropwise under the condition of stirring with a magnetic stirrer to control the dropping rate at 1ml / min. After the dropwise addition, seal the tube tightly and then control the temperature at 60°C for 12h to obtain the reaction solution , the reaction process was followed by thin-layer chromatography;
[0035] Described cuprous catalyst is CuBr; ...
Embodiment 2
[0050] A kind of preparation method of α aryl amidine imine derivative, its preparation process specifically comprises the following steps:
[0051] (1), add the cuprous catalyst of magnetic stirring bar and 0.05mmol (7mg) and the N-R1 of 0.5mmol (146mg) in 10ml sealed tube, the benzenesulfonamide substituted by R2, nitrogen replacement 3-5 times, then use Inject 0.5mmol (63mg) of R3-substituted terminal alkyne and 0.5mmol (99mg) R4-substituted sulfonyl azide into a 100ul microsyringe, and inject 2ml of organic solvent dichloromethane into it with a 2.5ml syringe under nitrogen protection. Then add 1 mmol (140 μL) additive dropwise under the condition of stirring with a magnetic stirrer to control the dropping rate at 1 ml / min. After the dropwise addition, seal the tube and tighten it, and then control the temperature at 60° C. to react for 12 hours to obtain a reaction solution. The progress of the reaction was followed by thin layer chromatography;
[0052] Described cuprou...
Embodiment 3
[0067] A kind of preparation method of α aryl amidine imine derivative, its preparation process specifically comprises the following steps:
[0068] (1), add the cuprous catalyst of magnetic stirring bar and 0.075mmol (10.5mg) and the N-R1 of 0.5mmol (146mg) in 10ml sealed tube, the benzenesulfonamide substituted by R2, nitrogen replacement 3-5 times, then Inject 1.5mmol (190mg) of R3-substituted terminal alkyne and 1.5mmol (296mg) R4-substituted sulfonyl azide with a 100ul microsyringe needle, and inject 2ml of organic solvent dichloromethane into it with a 2.5ml syringe under nitrogen protection , and then add 1mmol (140μL) additive dropwise under the condition of stirring with a magnetic stirrer to control the dropping rate at 1ml / min. After the dropwise addition, seal the tube tightly, and then control the temperature at 25°C for 12 hours to obtain a reaction solution , the reaction process was followed by thin-layer chromatography;
[0069] Described cuprous catalyst is ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com