Preparation method and application of double-targeting anti-tumor recombinant protein based on antibody and macropinocytosis
A recombinant protein, macropinocytosis technology, applied in antitumor drugs, targeting specific cell fusion, peptide/protein components, etc., to achieve the effect of strong tissue penetration, significant therapeutic effect, and good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
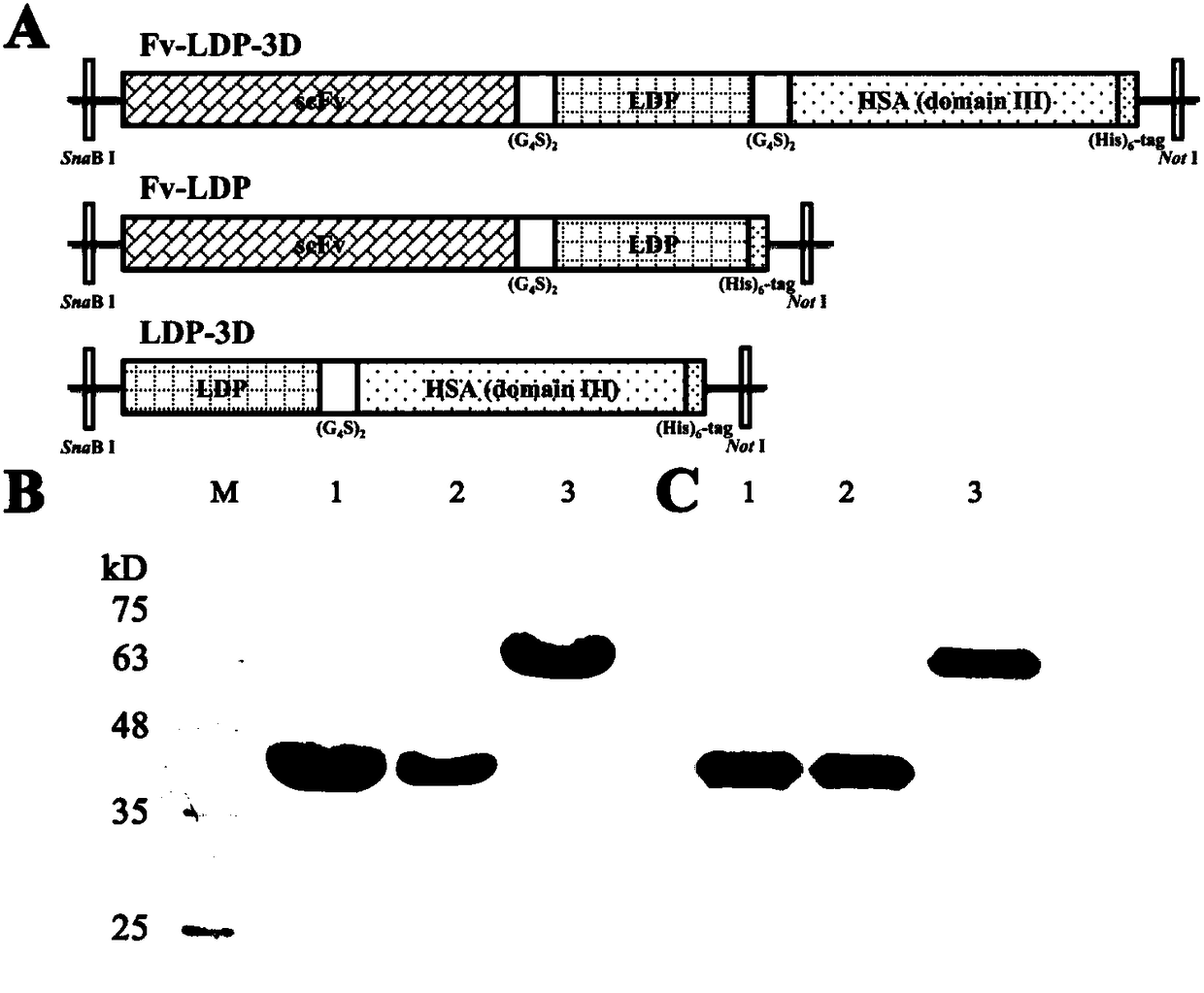
[0040] "Example 1" construction of recombinant expression vector pPIC9K-fv-ldp-3d
[0041] The complete structure of the recombinant protein Fv-LDP-3D is scFv-connecting peptide (G 4 S) 2 -LDP-connecting peptide (G 4S) 2 -3D, whose entire gene expression sequence was optimized and synthesized by GenScript based on the preferred codons of Pichia pastoris. The gene sequences of the experimental control recombinant proteins Fv-LDP and LDP-3D were constructed by designing primers using molecular biology techniques. Invitrogen TM Synthesized by the company, the expression vector is pPIC9K purchased from Invitrogen, and the Escherichia coli competent DH5α is a product of Genstar.
[0042] P1: 5'-TCTG TACGTA ATGGCCCAGGTCCAGCTTC-3' (the underline is the SnaB I restriction site)
[0043] P2: 5'-ATAAGAAT GCGGCCGC TTAGTGATGGTGATGG-3' (the underline is the Not I restriction site)
[0044] P3: 5'-TCTG TACGTA GCTCCAGCTTTCTCTG-3' (the underline is the SnaB I restriction site)
...
Embodiment 2
[0050] "Example 2" Expression and purification of recombinant protein in Pichia pastoris
[0051] The pPIC9K-fv-ldp-3d and the experimental control expression vectors pPIC9K-ldp-3d and pPIC9K-fv-ldp were transformed into Escherichia coli DH5α respectively, single clone strains were selected, and positive vector strains were confirmed by PCR identification and sequencing identification. The expression plasmid was extracted, linearized by Sal I enzyme digestion, and electrotransformed into Pichia pastoris GS115 competent cells after gel recovery. Pick a single colony for colony PCR identification, pick multiple His + The positive colonies were induced to express. After the expression conditions were optimized, the expression yield of the recombinant protein was detected, and the strain with higher expression was selected as the expression strain. The strain is named GS115-FL3, and was sent to the General Microorganism Center of China Microbiological Culture Collection Managemen...
Embodiment 3
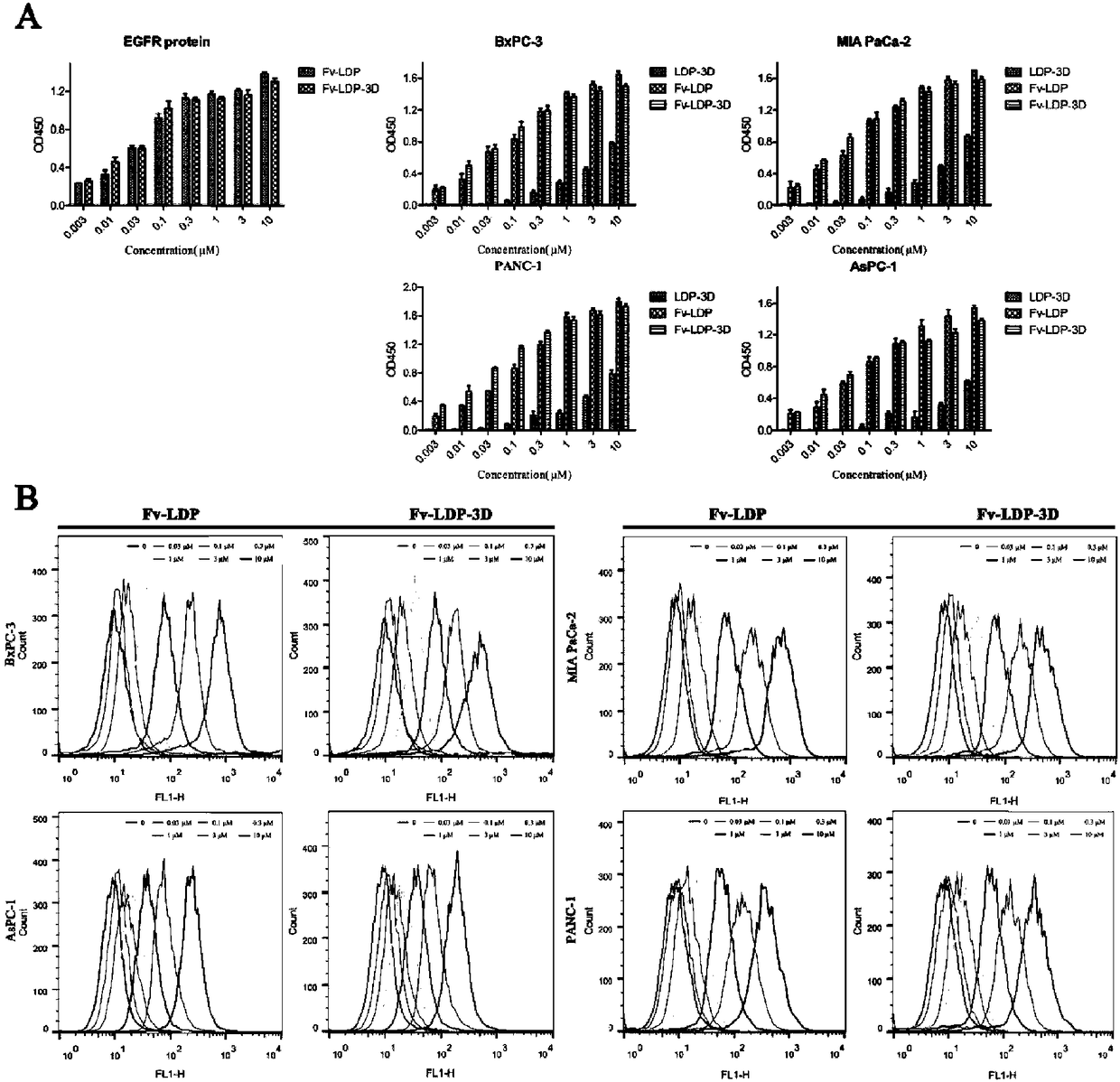
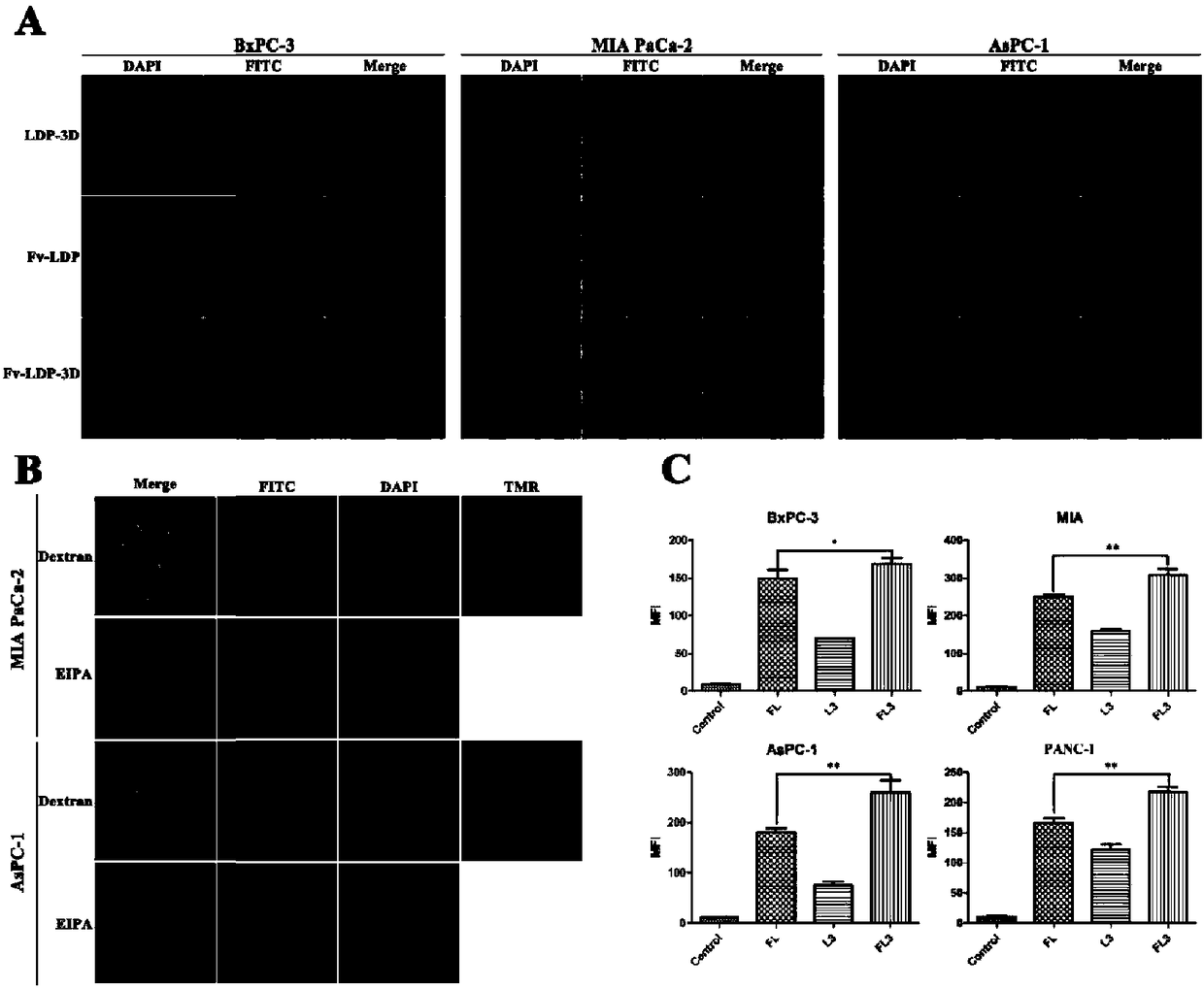
[0053] "Example 3" Recombinant protein and EGFR protein and tumor cell affinity activity analysis
[0054] 1. ELISA to detect the affinity activity of recombinant protein to EGFR protein and pancreatic cancer cells
[0055] EGFR protein powder was dissolved in PBS, diluted to 5 μg / ml, 50 μl / well was added to a 96-well plate, and coated overnight at 4°C. Rinse and wash with PBS for 3 times, discard the liquid in the well for later use; pancreatic cancer cells BxPC-3, MIA PaCa-2, AsPC-1 and PANC-1 were treated with 1×10 4 Cells / well density were seeded in a 96-well plate, cultured at 37°C for 24 hours, rinsed twice with PBS, added 50 μl / well of pre-cooled 0.05% glutaraldehyde at 4°C, fixed the cells at 4°C for 20 minutes, and fixed the cells Rinse with PBS 3 times, dry the residual liquid and set aside. The 96-well plate coated with EGFR and the cells fixed was blocked with 5% skimmed milk solution at 200 μl / well at room temperature for 2 hours; washed with PBST buffer (contai...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com