One-pot synthesis of copper-containing small-pore zeolites
A technology of zeolite and faujasite zeolite is applied in the field of preparing copper-containing small-pore zeolite, and can solve problems such as high energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] a) Synthesis of Cu-tetraethylenepentylamine complex (Cu-TEPA): 37.9g tetraethylenepentylamine (0.2 mole) was added to 50g CuSO 4 ·5H 2 O (0.2 mol) in 200g H 2 O in solution (1M solution), then kept stirring at room temperature for 2 hours.
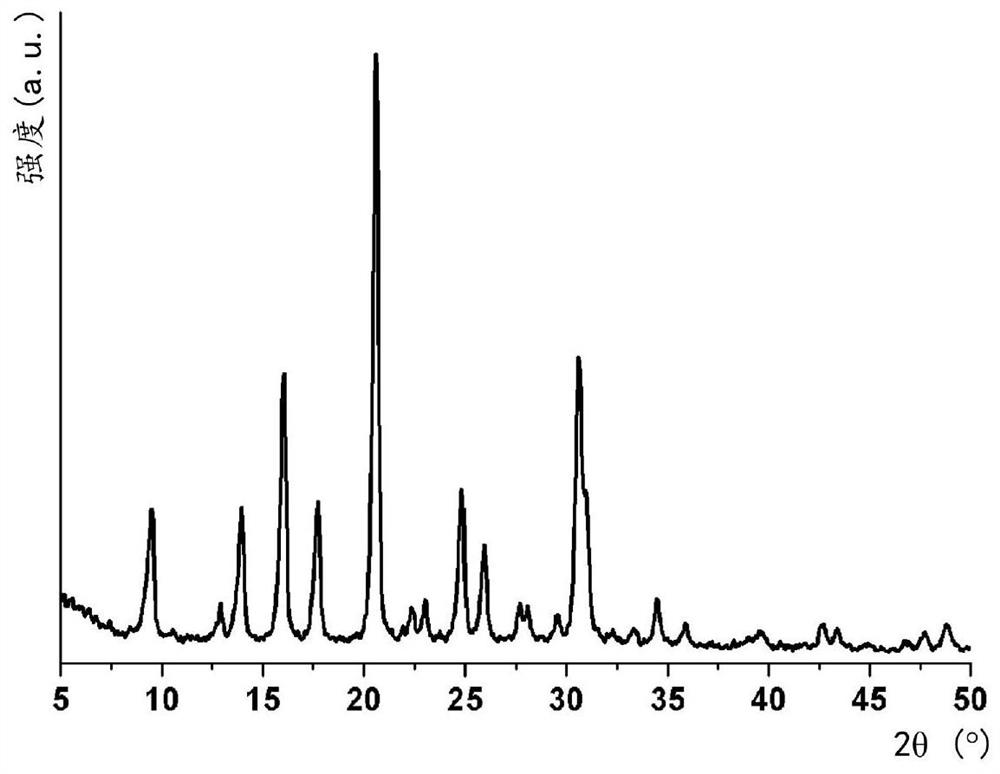
[0049] b) 3 g of SAR=30 (Si / Al=15) zeolite Y (CBV720 supplied by Zeolyst International) were suspended in 27 mL of 1.2 M sodium hydroxide solution. To this solution was added 1.5 mL of 1M Cu-TEPA solution. The final colloid has the following molar ratios: 1 SiO 2 / 0.033 Al 2 o 3 / 0.033 Cu-TEPA / 0.70 NaOH / 34H 2 O. The suspension was stirred at room temperature for 10 minutes before being transferred to an oven at 95 °C and left to stand for 7 days. After cooling to room temperature, the powder was isolated from the mother liquor by filtration, washed with deionized water and dried at 60 °C for 12 h. Generated zeolite according to the X-ray diffraction pattern (see figure 1 ) was determined to have a CHA framework type code...
Embodiment 2
[0052] 1.5 g of zeolite Y of SAR=30 (Si / Al=15) (CBV720 supplied by Zeolyst International) and 1.5 g of zeolite Y of SAR=5.1 (Si / Al=2.55) (CBV300 supplied by Zeolyst International) The mixture was suspended in 27 mL of 1.2 M potassium hydroxide solution. To this solution was added 3 mL of 1M Cu-TEPA solution. The final colloid has the following molar ratios: 1 SiO 2 / 0.10Al 2 o 3 / 0.076 Cu-TEPA / 0.82 KOH / 42H 2 O. The suspension was stirred at room temperature for 10 minutes before being transferred to an oven at 95 °C and left to stand for 5 days. After cooling to room temperature, the powder was isolated from the mother liquor by filtration, washed with deionized water and dried at 60 °C for 12 h. The resulting zeolite was determined to have a CHA framework type code with a Si / Al ratio of 3.3 and a CuO content of 6.4% by weight.
Embodiment 3
[0054] Example 2 was repeated except that instead of potassium hydroxide, 27 mL of 1.2M sodium hydroxide solution was disclosed. The final colloid has the following molar ratios: 1 SiO 2 / 0.10 Al 2 o 3 / 0.076 Cu-TEPA / 0.82 NaOH / 42H 2 O. The resulting zeolite after 11 days was determined to have a CHA framework type code with a Si / Al ratio of 2.9 and a CuO content of 8 wt%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com