Separation and purification method of cyclosporin H
A technology for the separation and purification of cyclosporine, which is applied in the field of biochemistry, can solve the problems of cumbersome purification and low yield, and achieve the effects of strong applicability, avoiding waste, and saving production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The crude cyclosporin H in Example 1 comes from the cross-over by-product of cyclosporin A purified by silica gel column chromatography, and the content of cyclosporin H was detected to be 10%.
[0035] The separation and purification method of the present embodiment comprises the following steps:
[0036] Preparation of liquid to be separated: Dissolve cyclosporine H crude product with 10% cyclosporin H content in 10 times the weight of methanol, filter to remove solid insoluble impurities to obtain a solution containing cyclosporine H, and press the solution volume Add water to 20% for stirring and diluting to make the liquid to be separated; stir and add 20% water to make the sample liquid contain water and the sample will not precipitate.
[0037] Chromatographic column preparation: Soak phenylcarbamate β-cyclodextrin silica gel in methanol and then install the column, then equilibrate the column with methanol with a volume percentage concentration of 40%; the diame...
Embodiment 2
[0043] The crude cyclosporin H in Example 2 was obtained from the fermentation broth of Trichoderma polyporus, and the cyclosporin A obtained by purifying cyclosporin A by multiple chromatography contained cross-linked impurities of cyclosporine, wherein the content of cyclosporin H was 30%.
[0044] The separation and purification method of the present embodiment comprises the following steps:
[0045] Preparation of liquid to be separated: Dissolve cyclosporine H crude product with 30% cyclosporine H content in acetonitrile of 10 times the weight, filter to remove solid insoluble impurities to obtain a solution containing cyclosporin H, and press the solution volume Add water to 15% and stir and dilute to make the liquid to be separated; stir and add a small amount of water to make the sample liquid contain water and the sample will not precipitate.
[0046] Chromatographic column preparation: Soak phenylcarbamate β-cyclodextrin silica gel in methanol and load the column, an...
Embodiment 3
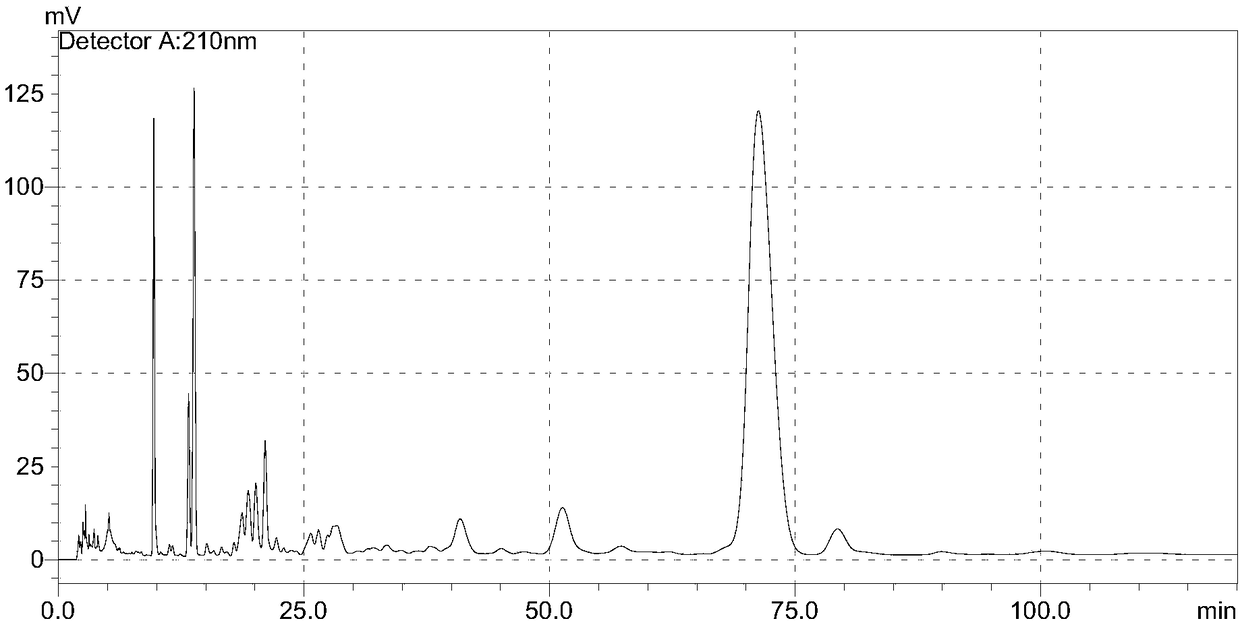
[0052] The crude cyclosporin H in Example 3 comes from cyclosporin H prepared by reacting cyclosporine A, and the content of cyclosporin H after pretreatment is 21.4%, and its HPLC figure is shown in Figure 5 , Background of the Invention The HPLC chart of the crude product containing cyclosporine H synthesized from cyclosporin A.
[0053] The separation and purification method of the present embodiment comprises the following steps:
[0054] Preparation of liquid to be separated: Dissolve cyclosporine H crude product with 50% cyclosporin H content in 8 times the weight of ethanol, filter to remove solid insoluble impurities to obtain a solution containing cyclosporine H, and press the solution volume Add water to 25% to stir and dilute to make the liquid to be separated; stir and add a small amount of water to make the sample liquid contain water and the sample will not precipitate.
[0055] Chromatographic column preparation: Soak phenylcarbamate β-cyclodextrin silica gel ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com