Influenza virus subunit vaccine purification method and applications thereof
A technology of subunit vaccine and influenza virus, applied in the field of preparation method and vaccine prepared by the method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] (1) Proliferation and concentration of virus
[0063] After diluting the working seed lot virus to a final concentration of 2.0lgEID50 / ml, inoculate the allantoic cavity of chicken embryos at a dose of 0.2ml / embryo, culture at 33°C for 48 hours, screen live chicken embryos, and place cold embryos at 4°C for 12 hours. Hours later, the allantoic fluid was harvested, and the allantoic fluid harvested from the single-type influenza virus was centrifuged and clarified and combined into a monovalent virus pooled liquid, and the monovalent virus pooled liquid was concentrated by ultrafiltration with a molecular weight cut-off ultrafiltration membrane of 1 million, and the concentration factor was 20 times .
[0064] (2) Purification before virus lysis
[0065] Prepare 15%, 30% and 60% sucrose solutions respectively, put the rotor into the ultracentrifuge, start and install the rotor, close the hatch, and set the speed at 1000rpm at the same time, after the rotor is stable, st...
Embodiment 2
[0075] A quadrivalent influenza virus subunit vaccine, each dose of the quadrivalent influenza virus subunit vaccine contains two lineage BY and BV strains of H1N1 type, H3N2 type and B type, and the content of each valent antigen is 30-36 μg / mL.
[0076] (1) Proliferation and concentration of virus
[0077] After diluting the batch of working seeds to a final concentration of 5.0lgEID50 / ml, inoculate the allantoic cavity of chicken embryos at a dose of 0.2ml / embryo, culture at 35°C for 72 hours, screen live chicken embryos, and place them in a cold place at 8°C. After 24 hours of embryos, the allantoic fluid was harvested, and the allantoic fluid harvested from the monotype influenza virus was centrifuged and clarified and combined into a monovalent virus pooled fluid. Use a 1 million molecular weight cut-off ultrafiltration membrane to conduct ultrafiltration and concentration of the monovalent virus pool solution, and the concentration factor is 60 times.
[0078] (2) Pu...
Embodiment 3
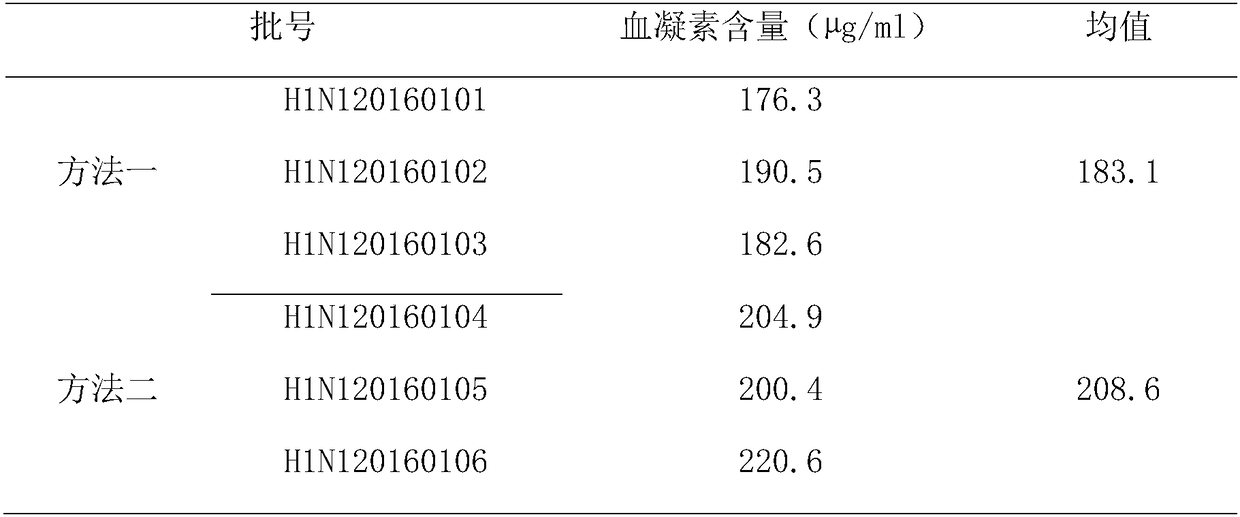
[0090] The influenza virus cracking electron micrograph of embodiment 3 different purification processes
[0091] 1. Materials:
[0092] H1N1 Influenza Virus Concentrate
[0093] 2. Method:
[0094] The concentrated solution of H1N1 influenza virus was lysed by two different virus purification processes.
[0095] The first is the pre-cleavage purification plus separate cleavage process adopted by the existing three-step purification method: first, the virus concentrate is subjected to sucrose density gradient centrifugation, and the absorbance value at 280nm is recorded with an ultraviolet spectrophotometer to detect the hemagglutination drops in each section degree, collect the target peak with high hemagglutination titer as the virus purification solution, and then add Triton N-101 lysing agent to the virus purification solution according to the final concentration of 1.0%. Samples were taken for electron microscopy examination.
[0096] The second is the pre-lysis purif...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com