High-selectivity 2-methyl allyl chloride synthesis method and synthesis reactor
A synthesis reactor and synthesis reaction technology, applied in chemical/physical/physicochemical reactors, chemical methods for reacting gaseous media with gaseous media, chemical instruments and methods, etc., can solve many side reactions, low selectivity, Unable to control the temperature and other problems, to achieve the effect of stable reaction process, improved reaction efficiency and high reaction selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
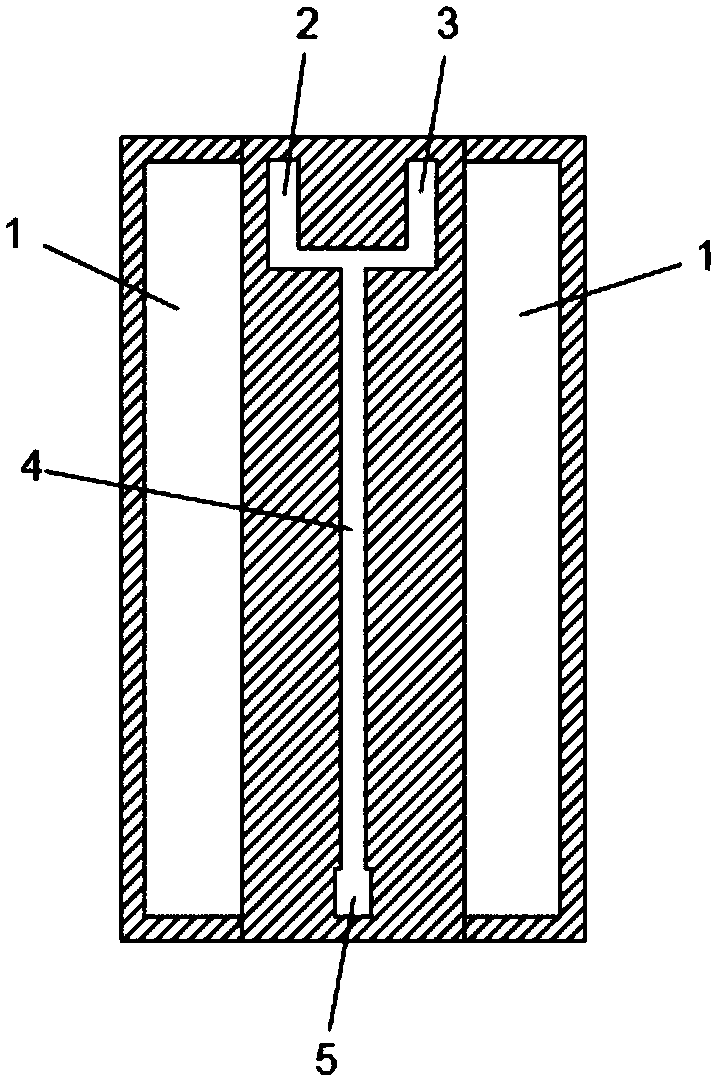
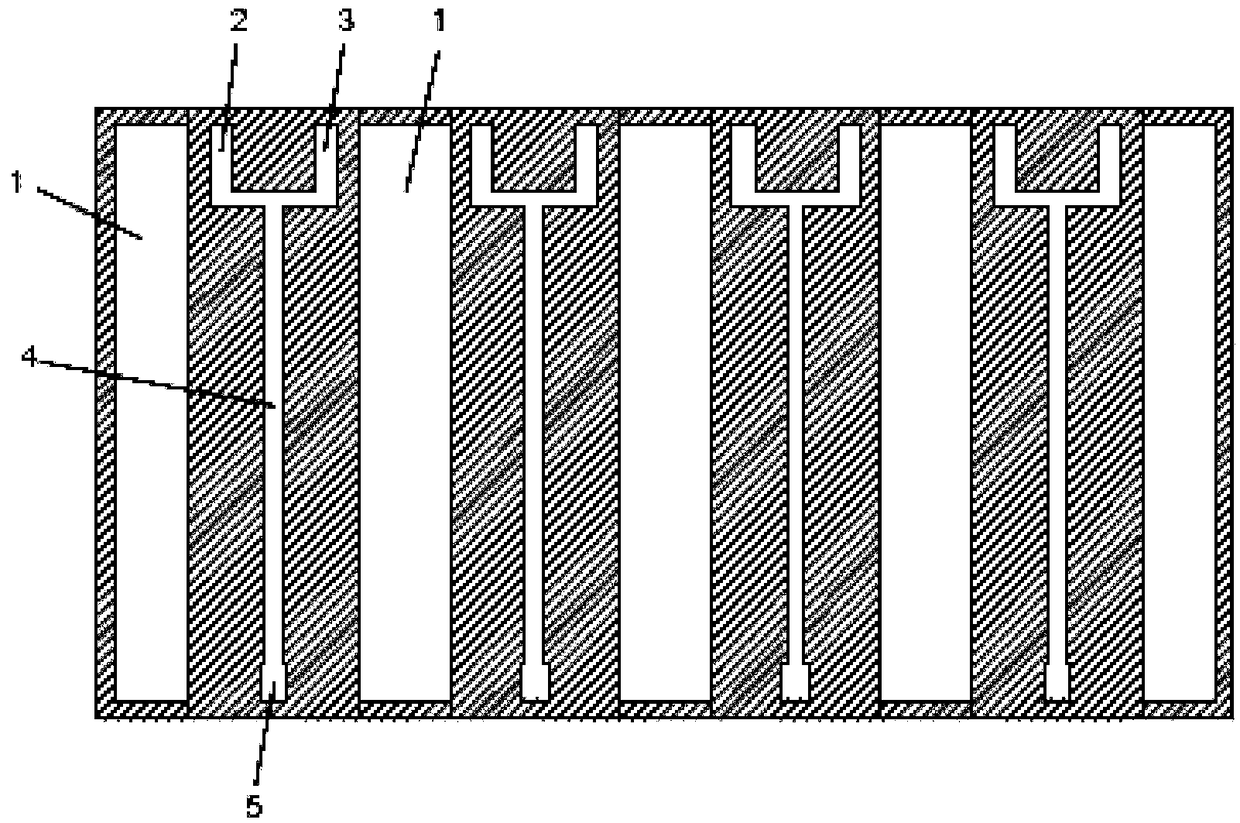
[0034] in such as figure 1 The microchannel reactor shown (the channel diameter is 0.2mm, and the heat exchange area calculated according to the actual reaction volume is 20000m 2 / m 3 ) into isobutene and chlorine respectively, by adjusting and controlling the flow of isobutene and chlorine, the reaction residence time reaches 1 second, the molar ratio of isobutene to chlorine is 1.005:1, and the reaction temperature is controlled by freezing brine to 0°C. After stable operation for 30 minutes, the composition of the liquid product was sampled and analyzed from the outlet of the reactor, and its mass content was: 2-methallyl chloride was 89.6%, chloro-tert-butane 2.3%, isobutenyl chloride 1.3%, dichloro-tert- Butane 5.6%, dichloroisobutene 1.2%; the selectivity of 2-methallyl chloride is calculated as 91.4%.
Embodiment 2
[0036] in such as figure 1 The shown microchannel reactor (the channel diameter is 0.5mm, and the heat exchange area calculated according to the actual reaction volume is 8000m 2 / m 3 ) into isobutene and chlorine respectively, by adjusting and controlling the flow of isobutene and chlorine, the reaction residence time reaches 0.1 second, the molar ratio of isobutene and chlorine is 1.02:1, and the reaction temperature is controlled by low temperature water to 30°C. After stable operation for 30 minutes, the composition of the liquid product was sampled and analyzed from the outlet of the reactor, and its mass content was: 2-methallyl chloride was 88.7%, chloro-tert-butane 2.1%, isobutenyl chloride 1.5%, dichloro-tert- Butane 6.0%, dichloroisobutene 1.5%; the selectivity of 2-methallyl chloride is 90.5%.
Embodiment 3
[0038] in such as figure 1 The shown microchannel reactor (the channel diameter is 0.4mm, and the heat exchange area calculated according to the actual reaction volume is 10000m 2 / m 3 ) into isobutene and chlorine respectively, by adjusting and controlling the flow of isobutene and chlorine, the reaction residence time reaches 0.5 seconds, the molar ratio of isobutene and chlorine is 1.01:1, and the reaction temperature is controlled by freezing brine to 10°C. After stable operation for 30 minutes, the composition of the liquid product was sampled and analyzed from the outlet of the reactor, and its mass content was: 2-methallyl chloride was 89.3%, chloro-tert-butane 2.3%, isobutenyl chloride 1.4%, dichloro-tert- Butane is 5.7%, dichloroisobutene is 1.3%, and the selectivity of 2-methallyl chloride is calculated to be 91.1%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com