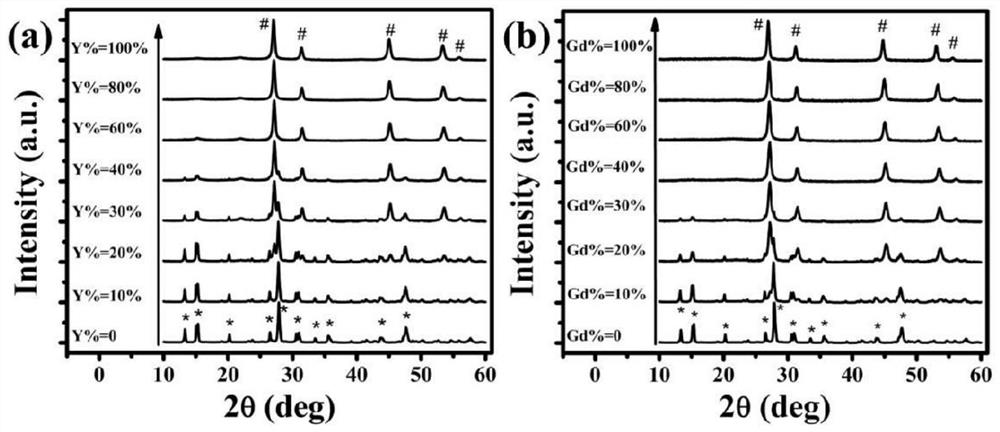
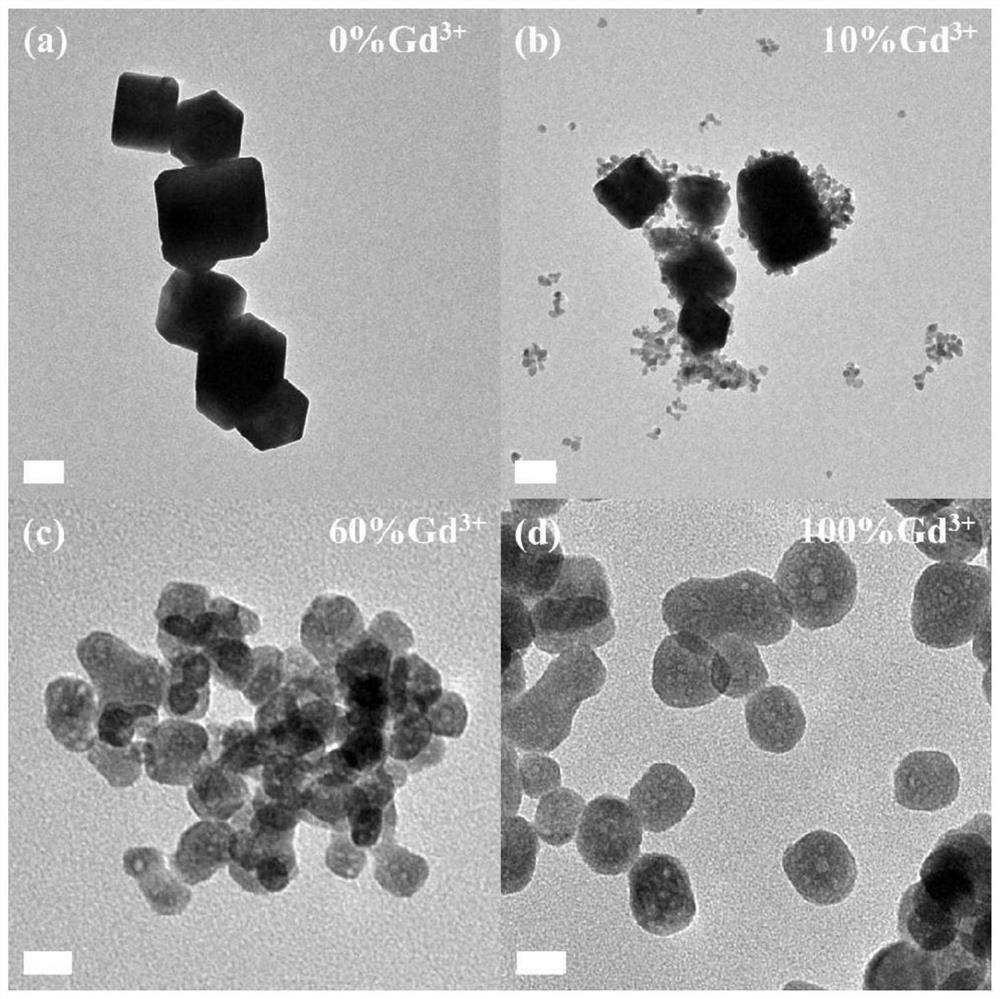
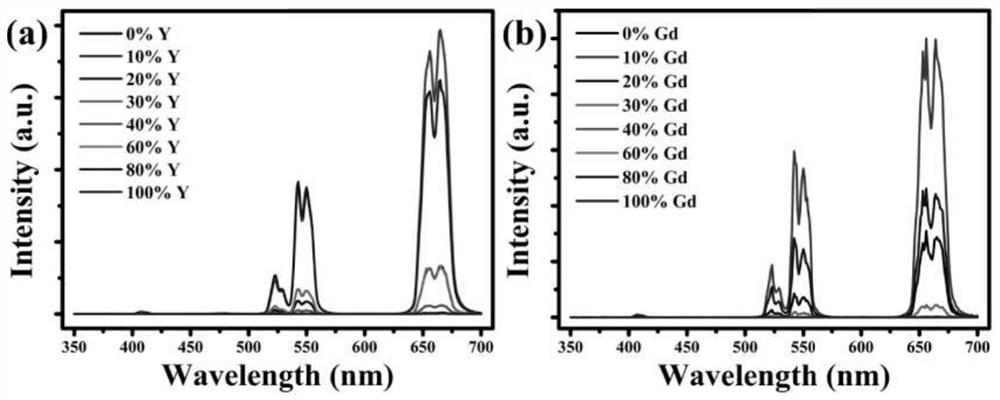
Potassium lutetium fluoride nanocrystal with delayed phase transition and greatly increased up-conversion luminescence intensity and preparation method thereof
A technology of potassium lutetium fluoride and luminous intensity, applied in the field of luminescent materials, can solve problems such as low luminous efficiency, reduced conversion luminescence, and phase transition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] 1. Prepare rare earth ion highly doped lutetium potassium fluoride nanocrystals by a water-based hydrothermal method. The specific steps are as follows:
[0046] (1) (0.8ml / 0.72ml / 0.64ml / 0.56ml / 0.48ml / 0.32ml / 0.16ml / 0ml) 1M lutetium nitrate solution, 0.36ml 0.5M ytterbium nitrate solution, 0.2ml 0.1M erbium nitrate solution , and (0ml / 0.08ml / 0.16ml / 0.24ml / 0.32ml / 0.48ml / 0.64ml / 0.8ml) 1M nitrate (Y 3+ / Gd 3+ ) solution was added to 10 ml of deionized water, and after magnetic stirring for 10 minutes, 3.75 ml of 0.4M dipotassium EDTA solution was added, and a white cloudy liquid was formed after 10 minutes under magnetic stirring, and finally 2.4 ml of 5M fluorine was added. Potassium chloride solution was added, and deionized water was added so that the total volume of all solutions was 30 ml, and a transparent colloid was formed after 30 minutes of magnetic stirring.
[0047] (2) Transfer the colloid to a stainless steel reactor with a liner for hydrothermal reaction. T...
Embodiment 2
[0052] 1. Prepare rare earth ion highly doped lutetium potassium fluoride nanocrystals by oil-based hydrothermal method. The specific steps are as follows:
[0053] (1) First, 2 ml of oleic acid, 10 ml of absolute ethanol and 4 mmol of potassium hydroxide were mixed and stirred for 10 minutes to form clear solution A. Add (0.8ml / 0.72ml / 0.64ml / 0.56ml / 0.48ml / 0.32ml / 0.16ml / 0ml) 1M lutetium nitrate solution, 0.36ml 0.5M ytterbium nitrate solution, 0.2ml 0.1M erbium nitrate solution, and ( 0ml / 0.08ml / 0.16ml / 0.24ml / 0.32ml / 0.48ml / 0.64ml / 0.8ml) 1M gadolinium nitrate solution was added to 12ml deionized water and stirred magnetically for 10 minutes to form solution B. Then solution A and solution B were mixed, and a white cloudy solution was formed after 10 minutes under magnetic stirring. Finally, 2.4 ml of 5M potassium fluoride solution was added, and deionized water was added so that the total volume of all solutions was 30 ml. A transparent colloid formed after 30 minutes of magne...
Embodiment 3
[0059] 1. Use oil-based hydrothermal method to prepare rare earth ion highly doped lutetium potassium fluoride nanocrystals at high temperature. The specific steps are as follows:
[0060] (1) First, 2 ml of oleic acid, 10 ml of absolute ethanol and 4 mmol of potassium hydroxide were mixed and stirred for 10 minutes to form clear solution A. Add (0.8ml / 0.72ml / 0.64ml / 0.56ml / 0.48ml / 0.32ml / 0.16ml / 0ml) 1M lutetium nitrate solution, 0.36ml 0.5M ytterbium nitrate solution, 0.2ml 0.1M erbium nitrate solution, and ( 0ml / 0.08ml / 0.16ml / 0.24ml / 0.32ml / 0.48ml / 0.64ml / 0.8ml) 1M gadolinium nitrate solution was added to 12ml deionized water and stirred magnetically for 10 minutes to form solution B. Then solution A and solution B were mixed, and a white cloudy solution was formed after 10 minutes under magnetic stirring. Finally, 2.4 ml of 5M potassium fluoride solution was added, and deionized water was added so that the total volume of all solutions was 30 ml. A transparent colloid formed a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com